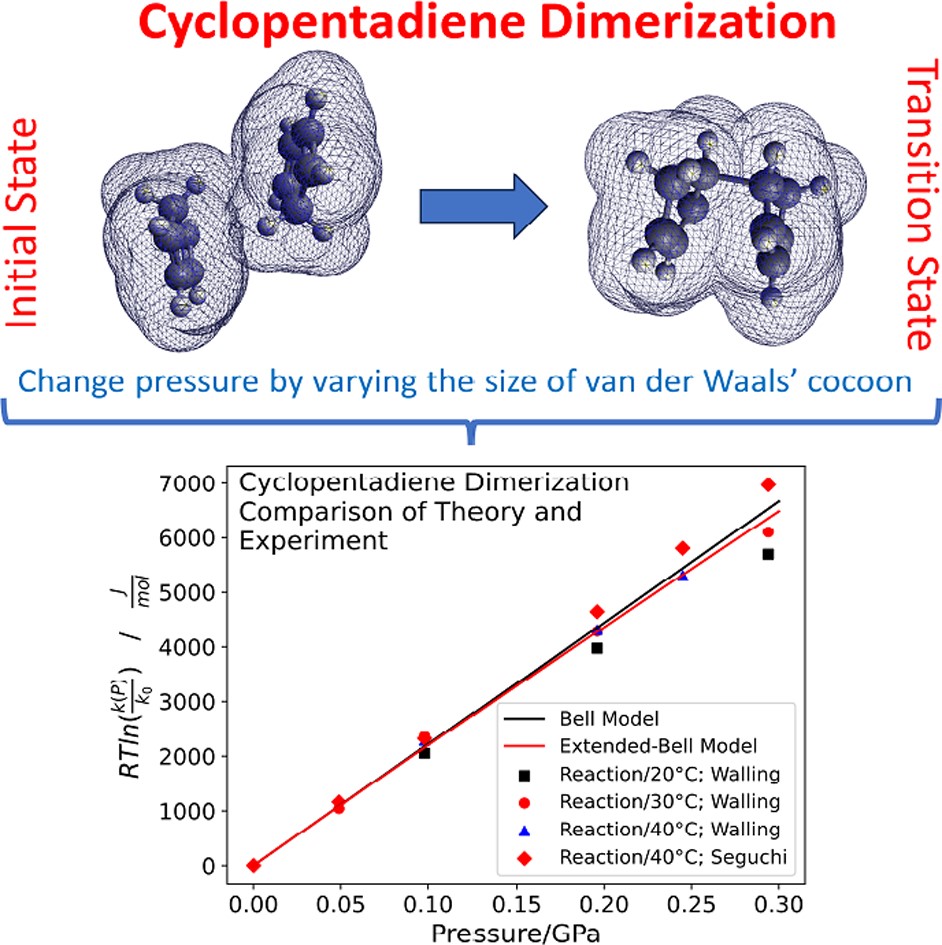
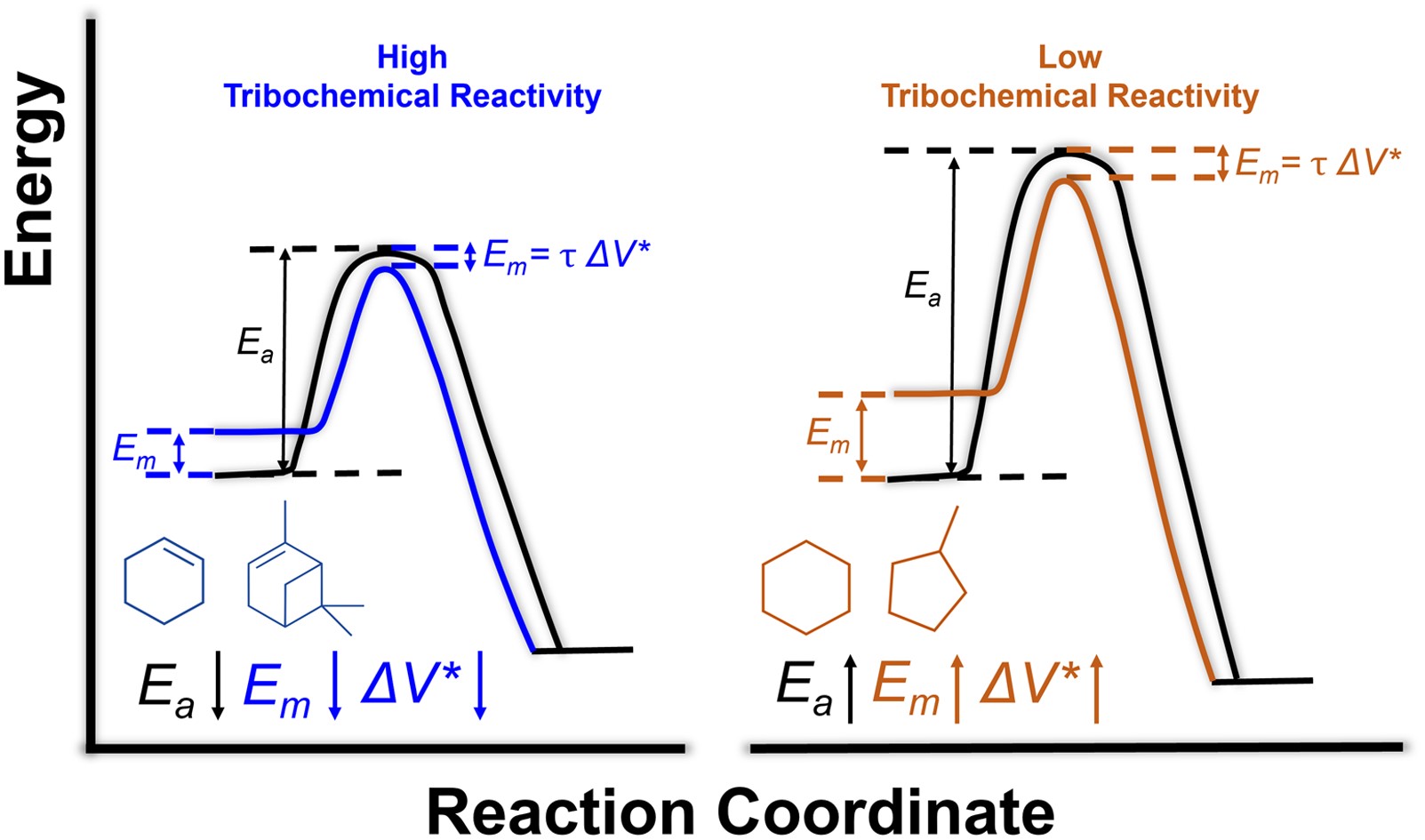
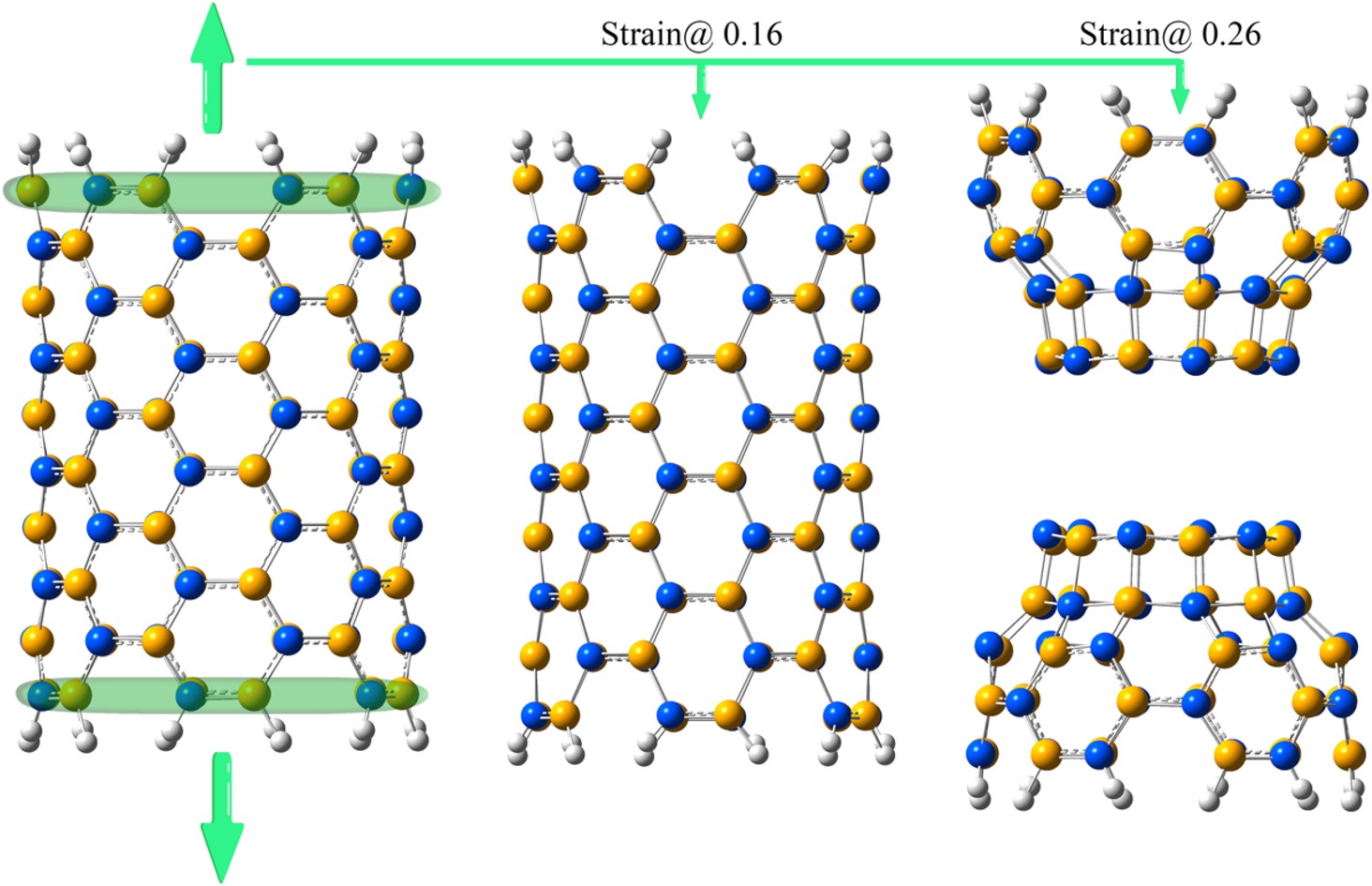
RSC Mechanochemistry has published its first articles. To celebrate this, we asked the authors to discuss their work in some more detail.
In this edition, we hear from Lars Borchardt about their study titled Cyanation of aryl halides using potassium hexacyanoferrate(II) via direct mechanocatalysis.
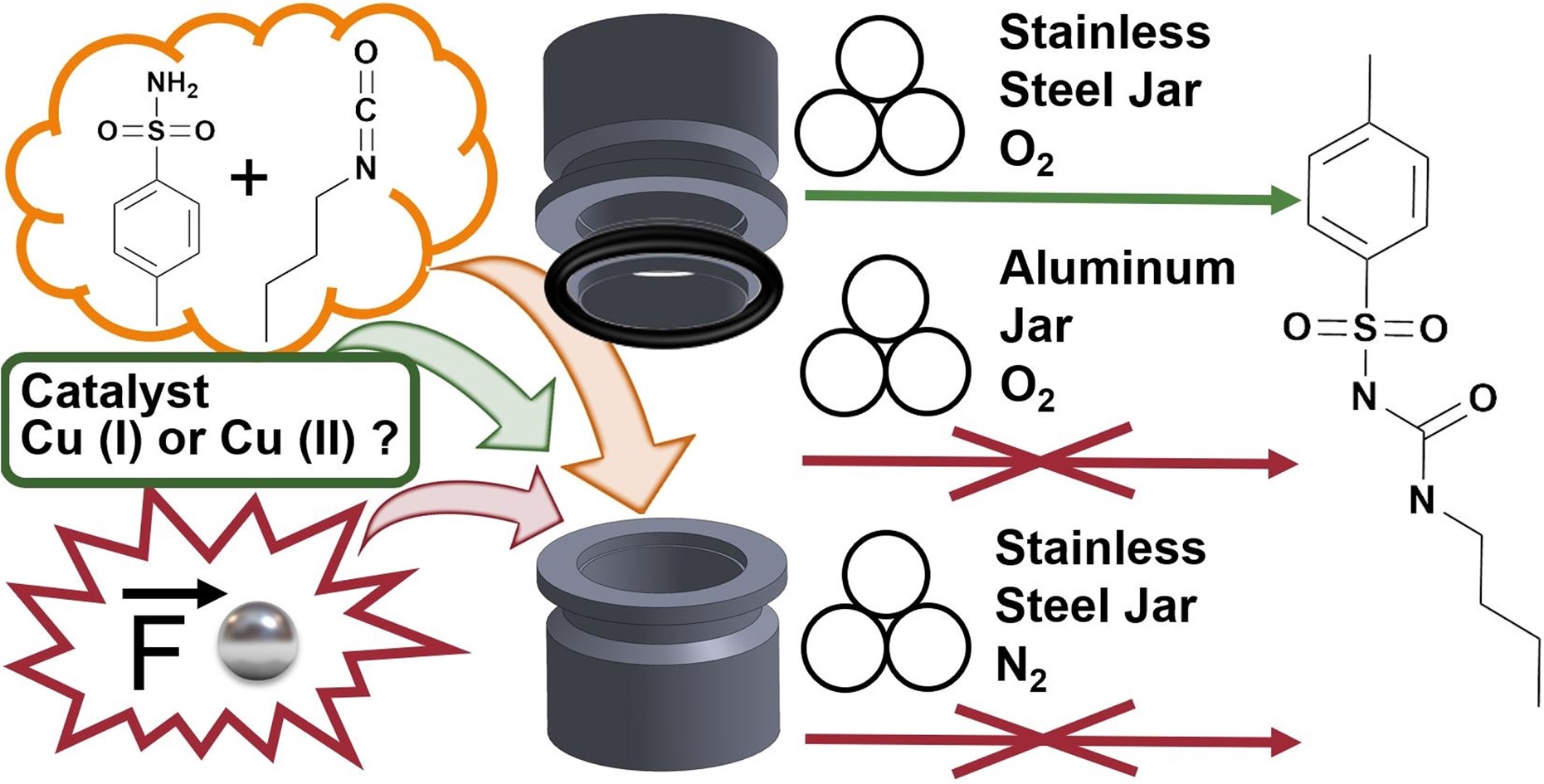
“Our mechanochemical approach allows for sustainable cyanation, delivering up to 90% yield without the use of solvents or toxic cyanide sources.”
“By leveraging potassium hexacyanoferrate (II) and a catalytically active Pd ball, we achieve a safer and environmentally friendly cyanation process with high efficiency.”
“This research underscores the power of mechanochemistry, offering a room temperature alternative to traditional cyanation methods that typically require high temperatures.”
“Our findings contribute to the growing field of green chemistry, showcasing a solvent-free, much safer alternative that doesn’t compromise on efficiency.”
“The research highlights the mechanistic complexity of the process, with XPS and PXRD analyses providing insights into the reaction mechanistic.”
Want to know more about their work? Read the full paper here!
Cyanation of aryl halides using potassium hexacyanoferrate(II) via direct mechanocatalysis
Suhmi Hwang, Phil M. Preuß, Wilm Pickhardt, Sven Grätz and Lars Borchardt
RSC Mechanochem. 2024, Advance Article, DOI: 10.1039/D4MR00054D
 |
RSC Mechanochemistry offers you an inclusive and dedicated home for the ideas, scientific language and approaches that cut across the many disciplines mechanochemistry touches. Here we are seeking to build knowledge, as well as foster innovation and discovery at this forefront of chemistry. Whether you are seeking to understand the fundamentals of mechanochemistry, or you are excited by its applications and potential, this journal is for you.
|


 In my field of soft matter mechanochemistry, we have seen some pretty exciting progress thanks to the development of increasing numbers of synthetic molecules that respond to mechanical force in specific ways. Some key examples are mechanochromic, mechanofluorescent and mechanoluminescent reporters, as well as force-triggered release mechanisms through mechanochemical linkers, reactions kick-started by radicals or mechanocatalysts, mechanochemical switches, and, more recently, artificial catch bonds. What is really exciting is that more and more of these mechanoresponsive systems are working in water, which opens up a lot of possibilities for integrating them with biological systems.
In my field of soft matter mechanochemistry, we have seen some pretty exciting progress thanks to the development of increasing numbers of synthetic molecules that respond to mechanical force in specific ways. Some key examples are mechanochromic, mechanofluorescent and mechanoluminescent reporters, as well as force-triggered release mechanisms through mechanochemical linkers, reactions kick-started by radicals or mechanocatalysts, mechanochemical switches, and, more recently, artificial catch bonds. What is really exciting is that more and more of these mechanoresponsive systems are working in water, which opens up a lot of possibilities for integrating them with biological systems. There is considerable evidence that mechanochemistry is often better than other synthetic methods, especially solution-based ones. Mechanochemistry uses mechanical force to drive chemical reactions, and it can be more efficient, resource-saving and environmentally friendly than traditional solutions.
There is considerable evidence that mechanochemistry is often better than other synthetic methods, especially solution-based ones. Mechanochemistry uses mechanical force to drive chemical reactions, and it can be more efficient, resource-saving and environmentally friendly than traditional solutions. Mechanochemistry has always been a paradigm-shifting method for conducting chemical reactions. While we often celebrate groundbreaking ideas in hindsight, they are not always embraced immediately. Consider Galileo Galilei, who faced life imprisonment for endorsing Copernicus’ theory that the Earth orbits the sun. Similarly, Alfred Wegener encountered not just skepticism, but outright hostility for proposing the concept of continental drift, suggesting that continents were once connected and moved across the Earth. One of Wegener’s detractors stated “It is certain the Wegener’s theory was established with a superficial use of scientific methods, ignoring the various fields of geology.” He continued to state “We can only try to keep our distance and beg him not to deal with geology any longer…” Even Einstein’s view of quantum physics was not all that favorable, famously stating “God does not play dice with the Universe” and describing what we now call quantum entanglement as “spooky action at a distance”. These examples highlight not just a mere clash of ideas, but also the hostility directed towards the individuals advocating them.
Mechanochemistry has always been a paradigm-shifting method for conducting chemical reactions. While we often celebrate groundbreaking ideas in hindsight, they are not always embraced immediately. Consider Galileo Galilei, who faced life imprisonment for endorsing Copernicus’ theory that the Earth orbits the sun. Similarly, Alfred Wegener encountered not just skepticism, but outright hostility for proposing the concept of continental drift, suggesting that continents were once connected and moved across the Earth. One of Wegener’s detractors stated “It is certain the Wegener’s theory was established with a superficial use of scientific methods, ignoring the various fields of geology.” He continued to state “We can only try to keep our distance and beg him not to deal with geology any longer…” Even Einstein’s view of quantum physics was not all that favorable, famously stating “God does not play dice with the Universe” and describing what we now call quantum entanglement as “spooky action at a distance”. These examples highlight not just a mere clash of ideas, but also the hostility directed towards the individuals advocating them. Mechanochemistry has the potential to revolutionise many industrial applications, such as energy, nanomaterials, and environmental remediation. By using mechanochemistry, our industry can potentially reduce their costs, waste, and environmental impact, while increasing their efficiency, quality, and innovation. Mechanochemistry can also enable the discovery of new compounds and mechanisms that are inaccessible by conventional methods.
Mechanochemistry has the potential to revolutionise many industrial applications, such as energy, nanomaterials, and environmental remediation. By using mechanochemistry, our industry can potentially reduce their costs, waste, and environmental impact, while increasing their efficiency, quality, and innovation. Mechanochemistry can also enable the discovery of new compounds and mechanisms that are inaccessible by conventional methods.