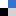Taking a historical snapshot of chiral plasmonics, after discovery in the 19th century and becoming more important in the 20th century for pharmacological and biological applications, in the early 2000s, twisted plasmonic nanostructures revealed strong chiroptical responses. However, their fabrication remained complex and limited. The 2010s introduced enabling tools such as DNA origami, colloidal self-assembly, and metamaterials, allowing precise control over chiral plasmonic architectures—but mostly within specialized labs.
The chiral plasmonic community experienced a major shift in the late 2010s, driven by easier access to chirality-related technologies: compact circularly polarized light (CPL) sources and affordable CD spectrometers made chiral plasmonics widely accessible. A recent visit to the Gold 2025 conference (San Sebastian, May 2025) revealed how dramatically the plasmonics community refocused from purely morphological anisotropic materials to nanomaterials with explicit chiral asymmetry. Across sessions – whether on sensing or catalysis – “chiral” appeared repeatedly in presentation titles.
The recent Focus article by Santiago and team published in Materials Horizons brings a new paradigm into fabrication techniques compared to previous approaches: what if chirality could be written post-synthetically by light? Their last years studies and other’s referred in this article, centering on light-to-matter chirality transfer, presents a conceptual leap. By exposing initially achiral plasmonic nanostructures to CPL, one can induce asymmetric surface transformations—effectively allowing the electromagnetic field to sculpt chirality dynamically. This “optical handwriting” of symmetry is not just elegant—it’s potentially more scalable, adaptive, and informative.

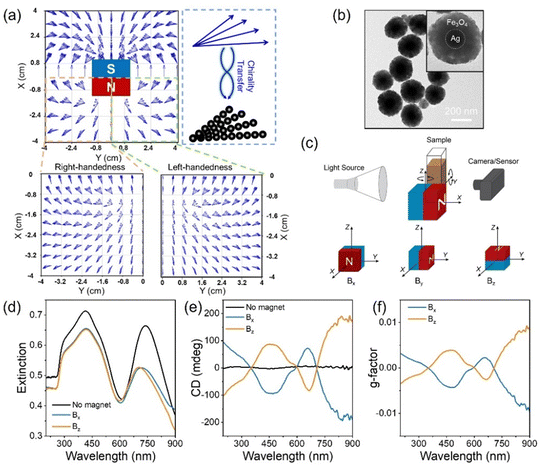
(a) Schematic diagram of the model for continuous CPL illumination of a colloidal Au nanocube, with the effective response being an average of six different directions for the propagation of light, and map of the differential rate of intraband hot carrier excitation under both polarizations of CPL. (b) Scheme of transformation of achiral nanobars into chiral ones in a colloidal suspension, driving galvanic replacement reaction CPL illumination of colloidal Au@Ag nanoprisms, alongside 3D models of the resulting geometries (obtained with TEM tomography), TEM images of the chiral bimetallic structures, and dissymmetry factor of the sample after illumination with CPL at 660 nm. (c) Geometrical analysis of the tomographic reconstruction of one of the resulting chiral structures (in blue), together with its mirror image (in red). Image and caption reproduced from Figure 12 (DOI= 10.1039/D5MH00179J) with permission from the Royal Society of Chemistry.
What makes this emerging direction so promising? First, light-induced chirality eliminates the need for chiral templates or reagents, granting full spatial and temporal control through illumination parameters – angle, polarization, phase structure, intensity. Second, the process doesn’t just modify geometry – it can modulate surface chemistry, reaction kinetics, or even local field helicity. It’s an enabler of materials previously considered inaccessible by static synthesis methods.
Despite these promises, the critical questions is a mechanistic pathways of chirality inscription under CPL. What’s the role of photothermal vs. hot-carrier effects? How does local field enhancement affect enantioselective growth? Quantitative metrics – such as dissymmetry factor (g) and CD spectral shifts—must be systematically correlated with structural evolution, not just endpoint characterization. Or even can chirality of these plasmonic structures transferred to chiral organic molecules? There is still a wide room for investigations.
To find out more, please read:
Light-to-matter chirality transfer in plasmonics
Eva Yazmin Santiago, Muhammad Irfan, Oscar Ávalos-Ovando, Alexander O. Govorov, Miguel A. Correa-Duarte and Lucas V. Besteiro
Mater. Horiz., 2025, DOI: 10.1039/D5MH00179J
About the blogger

Dr Olga Guselnikova is a member of the Materials Horizons Community Board. She joined the Center for Electrochemistry and Surface Technology (Austria) to work on functional materials as a group leader. Dr. Guselnikova received her PhD degree in chemistry from the University of Chemistry and Technology Prague (Czech Republic) and Tomsk Polytechnic University (Russia) in 2019. Her research interests are related to surface chemistry for functional materials. This means that she is applying her background in organic chemistry to materials science: plasmonic and polymer surfaces are hybridized with organic molecules to create high-performance elements and devices.
|