Electrification of transportation has become one of the most important central themes of modern society to build a sustainable system. The market for electric vehicles (EVs), which are powered by Li-ion batteries (LIBs), has been growing very rapidly and continuously. While EVs have become more popular on the road, there are concerns about their safety and somewhat limited driving ranges. In this respect, solid-state Li metal batteries have been extensively explored because of the following merits. First, solid-state electrolytes are more resistant to catching fire than liquid organic electrolytes that are flammable. Second, solid-state electrolytes are expected to be more stable against lithium metal dendrite penetration, which allows the use of energy-dense lithium metal anodes instead of graphitic carbon. However, it is still challenging to achieve “practical” high-energy density of solid-state Li metal batteries, which requires thick composite cathodes enabled by super-ionic conductors (>10 mS/cm). Although we have observed significant progress in improving the ionic conductivities of solid-state electrolytes, more fundamental studies to understand important parameters promoting lithium-ion conductivity and further exploration in new chemical spaces are needed.
A recent study by Han and colleagues developed a new borohydride/halide dual-substituted argyrodite-type solid-state electrolyte, which achieves an ionic conductivity of > 26 mS/cm after low-temperature sintering. They synthesized the borohydride/halide dual-substituted argyrodite solid-state electrolytes using a two-step ball-milling method from β-Li3PS4, LiBH4, and LiCl. While they also tried to incorporate Br– and I– instead of Cl– in the argyrodite solid-state electrolytes, they always produced unknown impurity phases and exhibited lower ionic conductivity. When they varied the compositions (2.5 ≥ x ≥ 1.5, Li3PS4 + xLiBH4 + 0.5LiCl), it was found that the composition of Li3PS4 + 2LiBH4 + 0.5LiCl (x = 2) exhibits the highest ionic conductivity of 16.4 mS/cm without sintering and 26.1 mS/cm after the sintering. They claimed that the increase of occupancy of BH4– in 4a may harm the ionic conductivity when x is larger than 2, but it still remains an open question why x = 2 composition has a sweet spot for the highest ionic conductivity in their system.
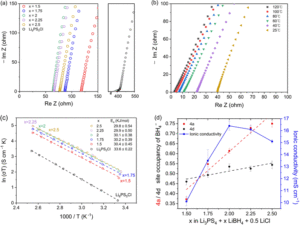
Figure 1: (a) Nyquist plots of Li3PS4 + xLiBH4 + 0.5LiCl (2.5 ≥ x ≥ 1.5) and commercial Li6PS5Cl. (b) EIS spectra of Li5.35PS4.35(BH4)1.15Cl0.5 at various temperatures. (c) Arrhenius plots and the activation energy of Li3PS4 + xLiBH4 + 0.5LiCl (2.5 ≥ x ≥ 1.5) and commercial Li6PS5Cl. (d) BH4- occupancy of 4a and 4d sites for each electrolyte and its ionic conductivity. The dashed lines are the trend lines of each site. Reproduced from DOI: 10.1039/D3MH01450A with permission from the Royal Society of Chemistry.
They further investigated the electrochemical properties, including oxidation stability limit, Li metal deposition/stripping cycles, and full cell tests. While they confirmed that their borohydride/halide dual-substituted argyrodite-type solid-state electrolytes are stable up to 5.0 V (vs. Li/Li+) using cyclic voltammetry (CV) technique, they used a simple cell configuration of Li-metal/solid-state electrolyte/current collector, which often overestimates oxidation limits. In the Li/solid-state electrolyte/Li symmetric cycling tests, they achieved stable cycling for up to 2000 hours (at 1mA/cm2, for 1 hour charge – 1 hour discharge cycles) and high critical current density (CCD) of 2.5 mA/cm2. They also confirmed the practical feasibility of their solid-state electrolytes in full-cell tests where Li metal and LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 are used as an anode and a cathode, respectively.
In conclusion, Han et al. demonstrated that the dual substitution of borohydride and halide enhances lithium-ionic conductivity of argyrodite-type solid-state electrolytes significantly. Their work sheds light on a new strategy of dual substitution to achieve super-ionic conductivity although there are remaining important questions to understand (i) why only Cl– could be soluble in the argyrodite structure along with borohydride and (ii) why a specific composition shows the best ionic conductivity. Answering the questions above will give us better insights to design even better super-ionic conductors in the future.
To find out more, please read:
Borohydride and halide dual-substituted lithium argyrodites
Ji-Hoon Han, Do Kyung Kim, Young Joo Lee, Young-Su Lee, Kyung-Woo Yi and Young Whan Cho.
Mater. Horiz., 2024, 11, 251-261, DOI: 10.1039/D3MH01450A
About the blogger

Haegyeom Kim is a Staff Scientist at Materials Sciences Division of Lawrence Berkeley National Laboratory (LBNL) and a Community Board member of Materials Horizons. In 2016-2019, he worked as a postdoctoral researcher at the LBNL (Supervisor: Prof. Gerbrand Ceder). He also spent 1 year as a postdoctoral researcher at Research Institute of Advanced Materials in Seoul National University (SNU) (Supervisor: Prof. Kisuk Kang) in 2015-2016. Dr. Kim completed his PhD degree in Materials Science and Engineering from SNU in 2015 (Advisor: Prof. Kisuk Kang), Master’s Degree in EEWS (Energy, Environment, Water and Sustainability) from Korea Advanced Institute of Science and Technology (KAIST) in 2011, and Bachelor’s Degree in Materials Science and Engineering from Hanyang University in 2009. At LBNL, Dr. Kim runs Renewable Energy Storage Lab, which designs and develops efficient and cost-effective energy storage and conversion materials based on the fundamental understanding of synthesis-structure-performance relationships. More information about Dr. Kim and his research group can be found here: https://kimhaegyeom1.wixsite.com/kim1 |