Patients with diabetes frequently develop chronic ulcers on their lower extremities that are extremely challenging to treat. To accelerate healing, numerous wound dressings have been developed to protect damaged tissue and possibly deliver therapeutics. However, few dressings can provide real-time monitoring of wound healing and patient health. Since diabetic ulcers are prone to persistent hyperglycemia, ischemia, prolonged inflammation, and bacterial infections, frequently assessing biomarkers is essential to optimize treatment and maximize healing outcomes.
In a recent work by Hou et. al., a multifunctional wound dressing is developed to not only shield diabetic foot wounds from insult and bacterial infections, but record wound site temperature, pH, and glucose levels. Additionally, the flexible electronic also monitors physiological signals such as patient heart rhythm and brain activity. To create the diagnostic dressing, a poly(ethylene glycol)-based polymer possessing cationic and anionic moieties, as well as the self-complementary hydrogen-bonding group, ureidopyrimidinone (UPy) is developed. The polymer abbreviated PADU after the first letter of each constituting monomer, forms flexible substrates due to intermolecular UPy interactions as well as ionic bonding between cationic and ionic segments.
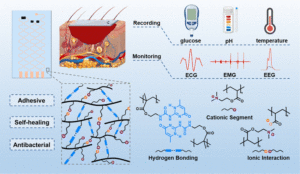
Figure 1: Schematics of using the diagnostic wound dressings, the internal molecular structure and corresponding performance of flexible polymeric substrates. Reproduced from DOI: 10.1039/D3MH02064A with permission from the Royal Society of Chemistry.
When applied to metals, rubber, plastic, glass, and biological tissues, PADU substrates display remarkable adhesion, notably exceeding an adhesive strength of 13 kPa for both diabetic and healthy mouse skin. Following damage to the patch, dynamic hydrogen bonding also endows PADU materials with self-healing capabilities, restoring 96% of their original mechanical strength after 24 hours. In practical use this gives PADU substrates a huge advantage because may be able to sustain electronic function despite physical damage from bodily movement.
In addition to its superb mechanical properties, PADU was also found to exhibit antibacterial activity. Killing of bacteria is achieved through disruption of the cell membrane by cationic groups within the polymer, making it active against even drug-resistant pathogens. Co-cultures with mammalian cells and blood cells, however, showed no cytotoxic or hemolytic effects, and biocompatibility was further confirmed by subcutaneous implantation into mice.
When glucose, pH, and temperature sensors were printed on PADU substrates, real-time monitoring of environmental conditions were reported with high accuracy. Gold foil electrodes could also be easily integrated into the patch to produce dressings capable of collecting electrocardiograms, electromyographic signals, and electroencephalograms. Due to their strong adhesion to skin, PADU-based sensors could even collect electrophysiological readings while patients were in motion, exceeding the accuracy of commercial sensors.
The dressing developed in the work by Hou, et. al. surpasses the capabilities of most diabetic wound dressings due its multifunctional performance as an adhesive and versatile electronic. Although initially designed for diabetic ulcers, the technology will likely find broad use as a diagnostic dressing for any type of slow-healing wound. In future studies, electronic patches may even be studied as implantable materials to monitor surgical site healing or internal injuries. Overall, the work demonstrates the vast utility of flexible bioelectronics and highlights the importance of developing new devices that not only treat but monitor chronic wounds.
To find out more, please read:
Skin-adhesive and self-healing diagnostic wound dressings for diabetic wound healing recording and electrophysiological signal monitoring
Zishuo Hou, Tengjiao Wang, Lei Wang, Junjie Wang, Yong Zhang, Qian Zhou, Zhengheng Zhang, Peng Li and Wei Huang
Mater. Horiz., 2024, Advance Article, DOI: 10.1039/D3MH02064A
About the blogger

Kelsey DeFrates is a Materials Horizons Community Board member. She received her Bachelor of Science in Bioengineering at Rowan University (Glassboro, New Jersey) in 2018 and completed her Ph.D. in Bioengineering through the University of California, Berkeley – UCSF joint graduate program in 2023. For her thesis research, Kelsey worked with Professor Phillip Messersmith at UC Berkeley on the development of supramolecular materials for drug delivery and tissue regeneration. In 2023, Kelsey joined Professor Christopher Hernandez at UCSF as a Chancellor’s Postdoctoral Fellow, where she is working to understand how bacteria sense and respond to physical stimuli. In future work, Kelsey hopes to use this information to develop engineered living materials for healthcare and sustainable building.
|