Peripheral nerve injury is a common complication of surgical procedures and traumatic insults that can result in severe discomfort including chronic pain and sensory defects. Ensuring that the injured nerve regenerates is therefore critical for the long-term well-being of affected individuals. A promising therapeutic strategy to enhance regeneration is to target both the initial inflammation and subsequently promote axonal regrowth. How to deliver drugs sequentially and on time for each patient is a complex engineering challenge requiring innovative solutions.
Toward this end, Shan and the team developed a stimuli-responsive drug delivery scaffold for a dual drug delivery that makes it possible to target both inflammation and axonal sprouting sequentially. The team created a composite material that consists of a Poly-L-lactic acid (PLLA) shell that provides mechanical support to the regenerating axons and that can be loaded with the novel bioactive and stimuli-responsive scaffold.
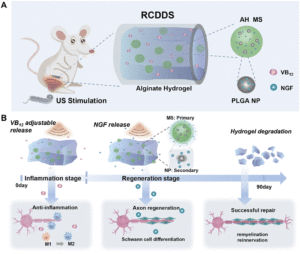
Figure 1. Schematic illustration of the structure and drug release process of the responsive cascade drug delivery scaffold (RCDDS) for peripheral nerve injury repair. (A) A brief illustration of the structure of the RCDDS implanted in SD rat. (B) Drug release process of the RCDDS and the corresponding repair stage. Vitamin B12 loaded in the hydrogel system can be adjustably released in the early stage by ultrasound to alleviate inflammation, while NGF loaded in alginate microspheres and PLGA nanoparticles can be gradually released from the RCDDS to promote axon regeneration one month after implantation. Reproduced from DOI: 10.1039/D3MH01511D with permission from the Royal Society of Chemistry
To achieve a staggered drug release profile, the team encapsulated vitamin B12 (vB12) with anti-inflammatory properties directly inside calcium crosslinked alginate hydrogel and used an additional multilevel encapsulation approach consisting of microspheres loaded with nerve growth factor (NGF)-adsorbed nanoparticles enabling their delayed release compared to vB12. The team then leveraged ultrasound as a stimulus to hierarchically open polymer chains in ultrasound-responsive calcium cross-linked alginate hydrogels. The rate of release of both vB12 and NGF was not predetermined and could be tuned by changing ultrasound intensity thus enabling the adjustment of the delivery profiles based on the individual patient’s healing progress. Interestingly, the ultrasound treatment alone improved peripheral nerve regeneration by promoting the secretion of neurotrophins in rodent models. Taken together, Shan and colleagues developed a novel strategy to promote nerve repair which utilizes ultrasound as an easily administrable stimulus that makes it possible to adjust the treatment of injured peripheral nerves based on patient’s needs.
To find out more, please read:
A responsive cascade drug delivery scaffold adapted to the therapeutic time window for peripheral nerve injury repair
Yizhu Shan, Lingling Xu, Xi Cui, Engui Wang, Fengying Jiang, Jiaxuan Li, Han Ouyang, Tailang Yin, Hongqing Feng, Dan Luo, Yan Zhang and Zhou Li
Mater. Horiz., 2024, Advance Article, DOI: 10.1039/D3MH01511D
About the blogger

Anna Stejskalova is currently a postdoctoral fellow at the Wyss Institute at Harvard Medical School and a member of the Materials Horizons Community Board. Dr. Stejskalova’s research focuses on female reproductive health and preterm birth utilizing organs on a chips and advanced biomaterials.
|