As we progress towards sustainable sources of energy, achieving efficient hydrogen generation through water splitting becomes increasingly vital. However, the challenges associated with electrode and membrane degradation due to corrosion in seawater hinder large-scale applications. While indirect seawater splitting through pre-desalination can circumvent corrosion issues, it introduces a drawback demanding additional energy input, rendering it economically less attractive. Therefore, the development of cost-effective water splitting electrocatalysts is essential for making the overall electrolysis process economically viable for widespread adoption and industrialization. Metal oxide electrocatalysts with active site engineering is a cutting-edge strategy to obtain high activity and durable catalysts for long-lasting performance under harsh saline conditions.
Recent work by Meng and team presents heterogeneous spin state molybdenum dioxide (MoO2) as a promising electrocatalyst to address the above critical challenges. Their novel approach involves the incorporation of Fe into MoO2 nanosheets on Ni foam (Fe-MoO2/NF) through a rapid carbothermal shocking method. This synthetic process facilitates the lattice dislocations, effectively exposing rich O vacancies and inducing a low-oxidation state in Mo sites, especially during the rapid Joule heating process. This results in the manipulation of spin states between Fe and Mo atoms. The resulting catalyst demonstrates remarkable performance for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in alkaline seawater.
The integration of heterogeneous spin states into MoO2 disrupts the d–d orbital coupling resulting in modified electronic configuration. This significantly affects the binding energy between the active sites and reaction intermediates, thereby enhancing the electrocatalytic activity. The catalytic activity of Fe–MoO2/NF is demonstrated by ultralow overpotentials recorded for both HER (17 mV@10 mA cm−2) and OER (310 mV@50 mA cm−2). Furthermore, the catalyst exhibits high selectivity in alkaline seawater splitting, showcasing its potential for efficient hydrogen production in challenging environmental conditions. To demonstrate the practical applicability of this newly developed electrocatalyst, the Fe–MoO2/NF is assembled into an anion exchange membrane seawater electrolyser that achieves a low energy consumption of 5.5 kW h m−3, emphasizing its practical application in renewable energy systems.
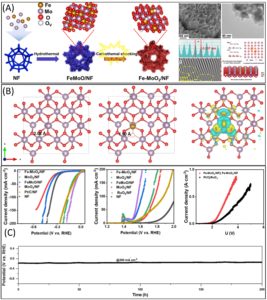
Figure 1: (A) Synthesis scheme of Fe–MoO2 /NF; SEM and TEM image along with HRTEM images and Schematic representations of lattice distortion formation mechanism of incorporated heterogeneous spin state (B) DFT analysis of the optimized models of MoO2, Fe–MoO2 and charge density difference plot of Fe–MoO2 (C) HER, OER and AEM electrolyzer polarization curves in saline water with long-term HER stability measurements in alkaline seawater solution. Reproduced from DOI: 10.1039/D3MH01757E with permission from the Royal Society of Chemistry.
An important aspect of this research is the successful coupling of Fe–MoO2/NF with a solar-driven electrolytic system, yielding a solar-to-hydrogen efficiency of 13.5%. This demonstrates the catalyst’s compatibility with solar energy, opening avenues for sustainable and clean hydrogen production. Theoretical insights into the electronic structure of Fe-incorporated MoO2, along with the abundance of oxygen vacancies, provides a deeper understanding of the catalytic mechanisms involved. Distortion of the Mo–O bonds, optimized through this method, plays a crucial role in enhancing the binding energy of adsorbed species during the electrochemical processes.
Looking forward, these findings hold significant promise for practical water splitting at a scalable level, especially in the context of solar-to-hydrogen production. The successful integration of Fe–MoO2/NF into a solar-driven electrolytic system is a critical step toward sustainable and eco-friendly widespread adoption and integration into large-scale hydrogen production systems. In the pursuit of practical water splitting for solar-to-hydrogen production on a larger scale, the key challenge lies in making the process economically viable and technologically feasible. Thus, the prospects for this work include optimizing the synthesis method and extending for other heterogeneous spin such as Ni and Co for seawater splitting, which might extend the versatility of the proposed strategy, offering a simple and efficient approach for efficient electrocatalysts. The simplicity and efficiency of the proposed strategy make it an attractive option for large-scale implementation. As the demand for green energy solutions and the reduction of carbon footprints continue to grow, the significance of advancements in water-splitting, especially saline water electrolysis driven by solar energy, cannot be overstated. This study adds to the growing body of evidence that renewable energy sources can be used to produce hydrogen, which will pave the ways towards a more greener and sustainable energy future.
To find out more, please read:
Rapid carbothermal shocking fabrication of iron-incorporated molybdenum oxide with heterogeneous spin states for enhanced overall water/seawater splitting
Jianpeng Sun, Shiyu Qin, Zhan Zhao, Zisheng Zhang and Xiangchao Meng
Mater. Horiz., 2024, Advance Article, DOI: 10.1039/D3MH01757E
About the blogger

Shahid Zaman is currently a postdoctoral fellow at Hydrogen Research Institute, University of Quebec Trois-Rivières, Canada, and he is a Materials Horizons Community Board member. He received his Ph.D. in Material Physics and Chemistry from Huazhong University of Science and Technology in 2021. From 2021 to 2023, he worked as a postdoctoral fellow at the Southern University of Science and Technology, China. Dr. Shahid’s current research interests are nanomaterials for electrocatalysis in proton exchange membrane fuel cells and water electrolyzers. |