Malaria is a parasitic disease caused by Plasmodium spp. which still ravages many parts of the world, responsible for killing an estimated 781,000 people each year according to the World Health Organisation’s 2010 World Malaria Report. Treatment is frequently associated with the development of resistance and so new drug leads are always needed.
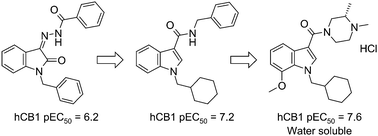
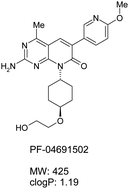
Dr Paul O’Neill and colleagues from the University of Liverpool and the London and Liverpool Schools of Tropical Medicine have developed a new series of tetraoxane analogues and screened them for their in vitro and in vivo antimalarial activity. All of the compounds synthesized showed remarkable in vitro activity in the low nanomolar range (0.2–3.7 nM) and several demonstrated promising oral activity in the P. berghei ANKA mouse model of malaria.
A preliminary study suggests that members of this series have improved metabolic stability compared with the parent compound RKA182 and these data coupled with the excellent activity profiles, low ClogP and high aqueous solubilities (e.g. >40mg/ml) make this series an exciting development in the struggle against malaria. Watch out for future studies on these compounds!
This HOT article is free to access, so read it today in MedChemComm
Second generation analogues of RKA182: synthetic tetraoxanes with outstanding in vitro and in vivo antimalarial activities
Francesc Marti, James Chadwick, Richard K. Amewu, Hollie Burrell-Saward, Abhishek Srivastava, Stephen A. Ward, Raman Sharma, Neil Berry and Paul M. O’Neill
Med. Chem. Commun., 2011, Advance Article
DOI: 10.1039/C1MD00102G










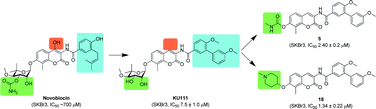
 The p53 signalling pathway is responsible for regulating the cell cycle, initiating DNA repair and triggering apoptosis where necessary. Its crucial importance can clearly be seen by the fact that about half of human cancers contain alterations in the p53 pathway. The proteins MDM2 and MDMX (a.k.a. MDM4) negatively regulate p53, and altered levels of these proteins are often deemed responsible for p53 alterations. As a result MDM2 has been extensively studied, but few MDM2 inhibitors have made it as far as clinical trials.
The p53 signalling pathway is responsible for regulating the cell cycle, initiating DNA repair and triggering apoptosis where necessary. Its crucial importance can clearly be seen by the fact that about half of human cancers contain alterations in the p53 pathway. The proteins MDM2 and MDMX (a.k.a. MDM4) negatively regulate p53, and altered levels of these proteins are often deemed responsible for p53 alterations. As a result MDM2 has been extensively studied, but few MDM2 inhibitors have made it as far as clinical trials. In this HOT paper David S. Millan and colleagues at Pfizer R&D explore the influence of intramolecular hydrogen bonding on the membrane permeability and bioavailability of some of these drugs. By determining the propensity of molecules to form intramolecular hydrogen bonds they are able to revise previous assumptions about where certain molecules sit in chemical space. They find that when hydrogen bonding is taken into account these drugs sit much closer to rule of five space – thereby explaining the successful absorption of drugs that otherwise violate rule of five principles.
In this HOT paper David S. Millan and colleagues at Pfizer R&D explore the influence of intramolecular hydrogen bonding on the membrane permeability and bioavailability of some of these drugs. By determining the propensity of molecules to form intramolecular hydrogen bonds they are able to revise previous assumptions about where certain molecules sit in chemical space. They find that when hydrogen bonding is taken into account these drugs sit much closer to rule of five space – thereby explaining the successful absorption of drugs that otherwise violate rule of five principles.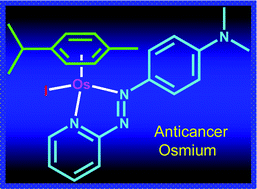 The majority of therapeutics clinically available for cancer treatment are platinum-based and work via a DNA alkylation mechanism that leads to cell apoptosis. But a number of cancers are resistant to apoptosis and not treatable with platinum-based drugs.
The majority of therapeutics clinically available for cancer treatment are platinum-based and work via a DNA alkylation mechanism that leads to cell apoptosis. But a number of cancers are resistant to apoptosis and not treatable with platinum-based drugs.