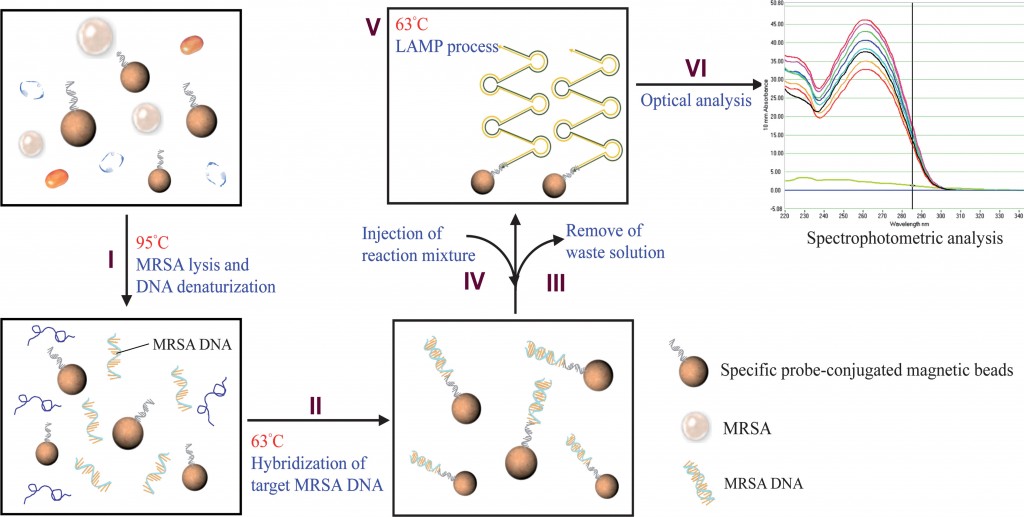
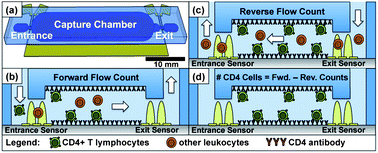
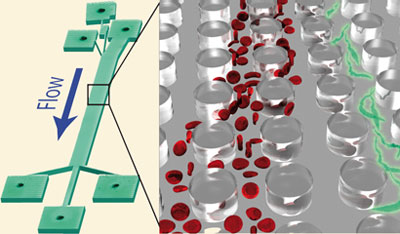
This month sees the following articles in Lab on a Chip that are in the top ten most accessed:-
Microfluidics and complex fluids
Ph Nghe, E. Terriac, M. Schneider, Z. Z. Li, M. Cloitre, B. Abecassis and P. Tabeling
Lab Chip, 2011, 11, 788-794, DOI: 10.1039/C0LC00192A, Tutorial Review
Rapid prototyping of microstructures in polydimethylsiloxane (PDMS) by direct UV-lithography
Tim Scharnweber, Roman Truckenmüller, Andrea M. Schneider, Alexander Welle, Martina Reinhardt and Stefan Giselbrecht
Lab Chip, 2011, 11, 1368-1371, DOI: 10.1039/C0LC00567C, Technical Note
Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP)
Hui-Sung Moon, Kiho Kwon, Seung-Il Kim, Hyunju Han, Joohyuk Sohn, Soohyeon Lee and Hyo-Il Jung
Lab Chip, 2011, 11, 1118-1125, DOI: 10.1039/C0LC00345J, Paper
nanoLAB: An ultraportable, handheld diagnostic laboratory for global health
Richard S. Gaster, Drew A. Hall and Shan X. Wang
Lab Chip, 2011, 11, 950-956, DOI: 10.1039/C0LC00534G, Paper
Programmed trapping of individual bacteria using micrometre-size sieves
Min-Cheol Kim, Brett C. Isenberg, Jason Sutin, Amit Meller, Joyce Y. Wong and Catherine M. Klapperich
Lab Chip, 2011, 11, 1089-1095, DOI: 10.1039/C0LC00362J, Paper
Lab-on-a-chip based immunosensor principles and technologies for the detection of cardiac biomarkers: a review
Mazher-Iqbal Mohammed and Marc P. Y. Desmulliez
Lab Chip, 2011, 11, 569-595, DOI: 10.1039/C0LC00204F, Tutorial Review
Simple room temperature bonding of thermoplastics and poly(dimethylsiloxane)
Vijaya Sunkara, Dong-Kyu Park, Hyundoo Hwang, Rattikan Chantiwas, Steven A. Soper and Yoon-Kyoung Cho
Lab Chip, 2011, 11, 962-965, DOI: 10.1039/C0LC00272K, Technical Note
Stand-alone self-powered integrated microfluidic blood analysis system (SIMBAS)
Ivan K. Dimov, Lourdes Basabe-Desmonts, Jose L. Garcia-Cordero, Benjamin M. Ross, Antonio J. Ricco and Luke P. Lee
Lab Chip, 2011, 11, 845-850, DOI: 10.1039/C0LC00403K, Paper
Integrated systems for rapid point of care (PoC) blood cell analysis
Cees van Berkel, James D. Gwyer, Steve Deane, Nicolas Green, Judith Holloway, Veronica Hollis and Hywel Morgan
Lab Chip, 2011, 11, 1249-1255, DOI: 10.1039/C0LC00587H, Paper
Affinity reagents for lab on chips
Mathias Uhlen and Helene Andersson Svahn
Lab Chip, 2011, Advance Article, DOI: 10.1039/C1LC90005F, Focus
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.
Comments Off on Top ten most accessed articles in February
![microtas2011_logo[1]](https://blogs.rsc.org/lc/files/2011/04/microtas2011_logo1.gif) Nominations are now invited for this award – the deadline for nominations is 20th May 2011. Full details of the criteria and how to submit the nominations are to be found on the Pioneers of Miniaturisation Lecture webpage which also provides more information about the award.
Nominations are now invited for this award – the deadline for nominations is 20th May 2011. Full details of the criteria and how to submit the nominations are to be found on the Pioneers of Miniaturisation Lecture webpage which also provides more information about the award.










 Another great issue of LOC is now available online, including Paul Vulto et al.‘s HOT article describing their new phaseguide technology for filling and emptying of microfluidic structures, independent of the chamber and channel geometry, which is highlighted on the
Another great issue of LOC is now available online, including Paul Vulto et al.‘s HOT article describing their new phaseguide technology for filling and emptying of microfluidic structures, independent of the chamber and channel geometry, which is highlighted on the  On the
On the 
 Featured on the
Featured on the  On the
On the Finally, the
Finally, the