Joong Yull Park, Sang-Hoon Lee and Shuichi Takayama
The International Symposium on Microchemistry and Microsystems 2011 (ISMM 2011), the Asian region forum on Micro Total Analysis Systems (μTAS), was held at a quiet hotel located in the southern part of Seoul, Korea, on June 2-4. Over 400 scientists and professionals arrived from diverse countries not only in Asia, but also from the U.S., the E.U., and beyond bringing with them their recent research advances and visions about the conference theme of: The Future of Miniaturized Systems.
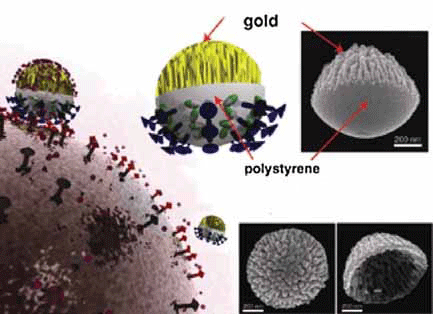
Figure 1. Nanocoral probes docking on a cancer-cell membrane. The highly roughened gold region of the nanocoral increases the molecular adsorption capacity and causes a strong surface-enhanced Raman spectroscopy signal.
The symposium opened with a plenary lecture from Professor Luke P. Lee, UC Berkely. Starting with a history of how stock market price increases paralleled commercialization of key technological breakthroughs, he predicted the future of miniaturized systems in terms of not only technology but potential financial impact as well. He emphasized the increasing need for quantitative biology and medicine and that harnessing micro/nano technology will be key. Considering that several decades were spent to move from vacuum tubes to microscale electronic components embedded in computers, he called upon both patience and hope for biochip and nanotechnology development. Specific research advances presented included Nano Satellites (Figure 1), an exciting concept for using nanoplasmonic particles to explore and visualize the inner space of living cells similar (both in concept and in terms of visual images obtained) to how macro-satellites help explore outer space.1 Combining the phenomenon of plasmon resonance, which is the collective resonant oscillation of electrons in a metal, with nanostructured particles, nanoplasmonic satellites focus and amplify light to nanometer-sized regions to shed new light on signaling pathways and cellular dynamics.
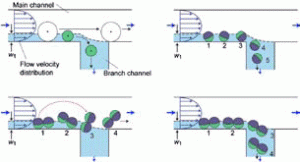
Figure 2. Size-dependent separation of spherical particles and possible rotation behaviors of spherical and nonspherical particles at the branch point.
The other plenary talks on this first day was given by Professor Minoru Seki, Chiba University, who introduced Pinched Flow Fractionation (PFF) and Hydrodynamic Filtration (HDF), a robust method to sort cells and particles by size in milliseconds (Figure 2).2 His presentation also covered calcium alginate gel fibers, anisotropic fibers, droplet-embedded fibers, and sandwich-type fibers generated in microfluidic chips. The anisotropic cross-sectional morphologies of the fibers are promising for guided growth of multiple cell types and thus for 2D/3D cell assembly which is required for tissue regeneration. His contributions are timely given the accelerating growth of cell-based therapies and associated increasing needs for cell sorting and analysis. Professor Bingcheng Lin, Chinese Academy of Sciences, proclaimed that we are ready for the future, to move microfluidics research to the next stage with increased applications, solving real-world problems, and realizing more commercialization. In the invited session, gold and silver nanoparticle-based colorimetric assay for protein and nucleic acids was presented by Professor Xiaodi Su, Institute of Materials Research and Engineering, Singarpore. The method utilizes metal nanoparticles’ unique optical properties and localized surface plasmon resonance (LSPR) for highly sensitive and lable-free detection.
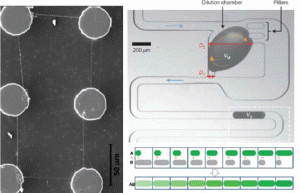
Figure 3. Left: ‘Suspended’ nanofibers connect six carbon posts. Right: Dilution module consists of input channel, dilution chamber, pillars, side channel, and output channel. Blue arrows indicate the flow direction, the dashed orange arrows the recirculatory mixing. Output droplets define a digital concentration gradient.
Plenary talks for the 2nd day were given by Professor Marc Madou, UC Irvine, and Andrew J. deMello, Imperial College London. Professor Madou introduced a way to combine photolithography, near-field electrospinning, and carbonization to pattern suspended carbon nanowire structures over incredible distances (left, Figure 3).3 These C-MEMS and C-NEMS structure were used for interdigitated electrode sensors, glucose sensors, bio-fuel cells and smart batteries. Keywords that describe Professor deMello’s lecture include ‘controlled droplet fusion’, ‘serial dilution on the microscale’, and ‘compartmentalization of single cell’. He showed a dilution module for high-throughput screening using droplet-based microfluidics (right, Figure 3), and demonstrated a homogeneous DNA-binding assay using this system. This digital concentration gradient.4 Other invited speakers included Professor Kahp-Yang Suh, Seoul National University, who introduced research opportunities in the area of ‘Body on a Chip’. One target organ he introduced was a kidney-on-a-chip. By combining microfluidics, advanced patterning technology called CFL (capillary force lithography), and multi-layer microfluidic device (MMD), a microfluidic bio-artificial kidney system was realized.5 The shear stress generated by flow in microfluidic channels is a necessary physiological stimulus for kidney cells along with hormonal stimulation. The biophysical and biochemical stimuli work synergetically to induce proper functioning of kidney cells in vitro. In other presentations, Professor Danny van Noort from the National University of Singapore, gave an aptly titled talk right before lunch about ‘Fish & Chips’. His micro fish tank array system (microaquarium) was cleverly designed to efficiently study fish embryo on a chip.
The last day of ISMM 2011 was opened by Professor Dong-Pyo Kim’s (Chungnam National University) plenary talk. He highlighted the importance of materials science and development. Despite the broad use of PDMS, particularly in academia, there is a critical need for alternate materials. For example, Professor Kim introduced various solvent resistant microreactors. He microfabricated PDMS, polyimid film and functional inorganic polymers by various lithographic techniques. The burgeoning field of microreactor technology is already starting to make a significant impact for industrial as well as benchside use, said Professor Kim.
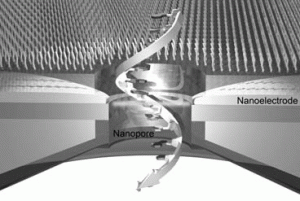
Figure 4. A single-molecule DNA translocates through a nanopore where a pair of nanoelectrodes fabricated to scan the tunnelling current across each nucleotide for label-free base sequence read-out.
Other highlights included description of the latest in single molecule DNA manipulation and sequencing technologies by Professors Kyubong Jo, Steven Soper and Tomoji Kawai. Professor Kawai, Osaka University, specifically introduced the next generation of DNA sequencing technology that utilizes gating nanopores. Two configurable nanoelectrodes enables the electrical detection of single nucleotides (Figure 4).6 Electron transport through single nucleotides occurs not by changes in the ionic current flowing parallel to the nanopore but by changes in the electric current flowing between the nanogap electrodes.
ISMM 2011 was organized in conjunction with the KBCS (Korea BioChip Society) with Professor Sang-Hoon Lee, Korea University serving as the general chair. The successful three days symposium closed on 4th June 2011. A cultural highlight of ISMM 2011 was a traditional Korean performance called Sa-mul-no-ri (Figure 5). ‘Sa-mul’ means ‘four objects’ (four traditional drums that represent thunder, wind, cloud, and rain) and ‘no-ri’ means ‘play’. The rapid and powerful drumbeat not only coordinated the dancing performers but also got the audience moving their bodies. This exciting and interactive performance of traditional music and dance symbolized well the vibrant field of miniaturized systems that we experienced at ISMM 2011 with fast-paced discoveries, cross-disciplinary interactions, and increasing collaboration with ‘audiences’ outside the micro/nanotechnology ‘performers’ including industry. It also felt like the rumblings of the exciting future where ‘play’ between the ‘four objects/subjects’ of medicine, chemistry, biology, and engineering opens unprecedented opportunities.

Figure 5. Dancing to the rhythm of Korean drums at ISMM 2011.
References
1 L. Y. Wu, B. M. Ross, S. Hong and L. P. Lee, Small, 2010, 6, 503-507.
2 S. Sugaya, M. Yamada and M. Seki, Biomicrofluidics, 2011, 5, 24103.
3 G. S. Bisht, G. Canton, A. Mirsepassi, L. Kulinsky, S. Oh, D. Dunn-Rankin and M. J. Madou, Nano Lett, 2011, 11, 1831-1837.
4 X. Niu, F. Gielen, J. B. Edel and A. J. deMello, Nat. Chem., 2011, 3, 437-442.
5 K. J. Jang and K. Y. Suh, Lab Chip, 2010, 10, 36-42.
6 M. Tsutsui, M. Taniguchi, K. Yokota and T. Kawai, Nat. Nanotechnol., 2010, 5, 286-290.
Comments Off on Rumblings of the big future of small chips heard in Asia












![c1lc20014c-image_250_tcm18-207329[1]](https://blogs.rsc.org/lc/files/2011/09/c1lc20014c-image_250_tcm18-20732912.jpg)






