You might have already seen our themed issue on optofluidics but in case you haven’t, here are the HOT articles from this issue:

Yi-Chung Tung et al. review the recent advances in optofluidic technologies that will open up new possibilities for on-chip phenotyping
Optofluidic detection for cellular phenotyping
Yi-Chung Tung, Nien-Tsu Huang, Bo-Ram Oh, Bishnubrata Patra, Chi-Chun Pan, Teng Qiu, Paul K. Chu, Wenjun Zhang and Katsuo Kurabayashi
DOI: 10.1039/C2LC40509A

Kevin Raymond et al. have developed an ‘optofluidic nose’ for sensing organic liquids based on wetting in photonic-crystal arrays.
Combinatorial wetting in colour: an optofluidic nose
Kevin P. Raymond, Ian B. Burgess, Mackenzie H. Kinney, Marko Lončar and Joanna Aizenberg
DOI: 10.1039/C2LC40489C

Haiwang Li and colleagues demonstrate the design of a bi-concave lens to perform both light focusing and diverging in-plane.
An electrokinetically tunable optofluidic bi-concave lens
Haiwang Li, Chaolong Song, Trung Dung Luong, Nam-Trung Nguyen and Teck Neng Wong
DOI: 10.1039/C2LC40406K

Yasutaka Hanada and coworkers show how to create a highly sensitive optofluidic chip for biochemical liquid assays by coating microfluidic channels with a low refractive index polymer and use of an optical waveguide.
Highly sensitive optofluidic chips for biochemical liquid assay fabricated by 3D femtosecond laser micromachining followed by polymer coating
Yasutaka Hanada, Koji Sugioka and Katsumi Midorikawa
DOI: 10.1039/C2LC40377C
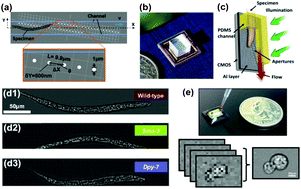
Jigang Wu, Guoan Zheng and Lap Man Lee focus on compact systems in their review of optical imaging techniques that can be integrated with microfluidics.
Optical imaging techniques in microfluidics and their applications
DOI: 10.1039/C2LC40517B
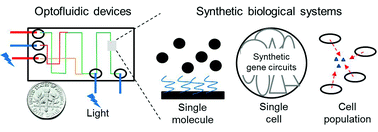 Chao-Min Cheng and colleagues provide thoughtful insight into the application of optofluidics to synthetic biology in this forward-looking Frontier article.
Chao-Min Cheng and colleagues provide thoughtful insight into the application of optofluidics to synthetic biology in this forward-looking Frontier article.
Frontiers of optofluidics in synthetic biology
Cheemeng Tan, Shih-Jie Lo, Philip R. LeDuc and Chao-Min Cheng
DOI: 10.1039/C2LC40828G
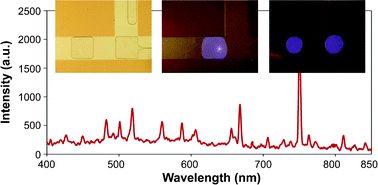
Shih-Kang Fan’s team manipulate 200 nL bubbles with DEP and ignite microplasma within them, with potential for future applications in the biomedical field.
Atmospheric-pressure microplasma in dielectrophoresis-driven bubbles for optical emission spectroscopy
Shih-Kang Fan, Yan-Ting Shen, Ling-Pin Tsai, Cheng-Che Hsu, Fu-Hsiang Ko and Yu-Ting Cheng
DOI: 10.1039/C2LC40499K
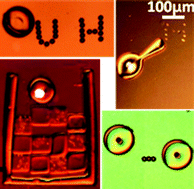 Wenqi Hu, Kelly S. Ishii, Qihui Fan and Aaron T. Ohta report a hydrogel microrobot which can be manipulated by laser-induced bubbles. Single or pairs of robots are able to assemble polystyrene beads and yeast cells into patterns.
Wenqi Hu, Kelly S. Ishii, Qihui Fan and Aaron T. Ohta report a hydrogel microrobot which can be manipulated by laser-induced bubbles. Single or pairs of robots are able to assemble polystyrene beads and yeast cells into patterns.
Hydrogel microrobots actuated by optically generated vapour bubbles
Wenqi Hu, Kelly S. Ishii, Qihui Fan and Aaron T. Ohta
DOI: 10.1039/C2LC40483D
Remember, all our cover articles are free to access for 6 weeks, and our HOT articles for 4 weeks. All you need to access them is an RSC Publishing Personal Account – signing up is quick and easy.
Comments Off on Hot articles in optofluidics



























