In June 2015, our most downloaded Lab on a Chip articles were:
Interesting read? Let us know your thoughts below.
And remember, you can submit directly to Lab on a Chip!
In June 2015, our most downloaded Lab on a Chip articles were:
Interesting read? Let us know your thoughts below.
And remember, you can submit directly to Lab on a Chip!
an article by Claire Weston, PhD student at Imperial College London
For effective treatment of many illnesses, in particular cancer, long-term monitoring of certain biomarkers is required. A needle probe with a chemically sensitive tip can be used, however this is invasive as it has to be inserted every time a new measurement is taken. There are also issues with tissue heterogenicity, as it is possible that changes in the measurements are solely due to a different local environment within the tissue.
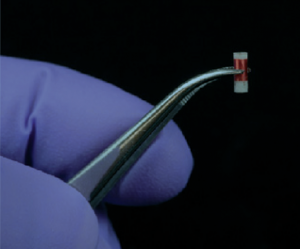
Size of sensor
Previously the Cima lab at MIT reported an improved alternative for long-term in vivo monitoring. A capsule containing an NMR contrast agent was inserted in vivo and measurements were recorded using an MRI scanner. They have now done one better and eliminated the need of very costly MRI equipment by developing a small NMR sensor that simply requires a small external reader coil.
Both the sensor and reader contain a circuit with a coil and when both are in range magnetic inductance occurs, causing field amplification inside the chamber of the sensor. This effectively means that a reading is taken only from tissue within the sensor, rather than surrounding tissue, and this is responsible for the high selectivity seen.
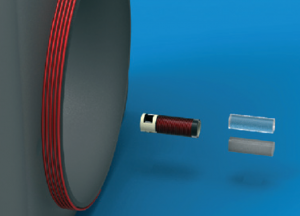
Different components of the system
In order to test their wireless sensor, Cima and coworkers separately measured pH and oxygen tension, both in vivo and in vitro. For the pH experiments, a polymer gel was used as the NMR contrast agent that had an exchangeable H atom with an appropriate pKa value. Using a tumor mouse model, pH readings were found to be lower when the sensor was nearer the tumor, as expected from the acidic nature of tumors. For the oxygen experiments, silicone was used as the contrast agent. The paramagnetic nature of molecular oxygen alters the relaxation time and this can therefore be used to determine the concentration of oxygen in the sensor.
From the success of their experiments, the authors conclude that they have demonstrated the flexibility of the sensor with these two measurements, and indeed there is huge potential for this NMR probe to greatly simplify in vivo monitoring.
To download the full article for free* click the link below:
Miniaturized, biopsy-implantable chemical sensor with wireless, magnetic resonance readout
C. C. Vassiliou, V. H. Liu and M. J. Cima
Lab Chip, 2015, 15, 3465-3472
DOI: 10.1039/C5LC00546A
—————-

About the webwriter
Claire Weston is a PhD student in the Fuchter Group, at Imperial College London. Her work is focused on developing novel photoswitches and photoswitchable inhibitors.
—————-
*Access is free until 01/10/2015 through a registered RSC account.
Flow cytometry is used to examine blood cells, which requires hydrodynamic sheath flow alignment and fluorescence antibody labelling, making it time-consuming and expensive. Advanced light scattering techniques (such as digital holography) are often seen as suitable alternatives, as they provide fast and label-free measurements.
In a recent Lab On A Chip article, Netti et al. from the Italian Institute of Technology, in collaboration with scientists from Germany and Russia, presented a camera-based light scattering approach, coupled with a viscoelasticity -induced cell migration technique. This new system is used to characterise the morphological properties of erythrocytes in microfluidic flows.
They obtained light scattering profiles (LSPs) of individual living cells in microfluidic flows over a wide angular range and matched them with scattering simulations to characterise their morphological properties. A healthy erythrocyte diameter lies between 6 and 9 µm. The diameter values obtained from the experiment lie between 7 and 8.3 µm, which is in good agreement with the existing literature.
‘The results demonstrate the ability of a rapid and cost effective way to measure the average dimensions of an erythrocyte population which can be easily related to the health of a patient,’ concludes Netti.
To gain deeper insight into LSP acquisition and simulation, you can read the full article for free* by following the link below.
Optical signature of erythrocytes by light scattering in microfluidic flows
D. Dannhauser, D. Rossi, F. Causa, P. Memmolo, A. Finizio, T. Wriedt, J. Hellmers, Y. Eremin, P. Ferraro and P. A. Netti
Lab Chip, 2015,15, 3278-3285
DOI: 10.1039/C5LC00525F
an article by Claire Weston, PhD student at Imperial College London
Researchers from the Kaneko Higashimori Lab at Osaka University and the Arai Lab at Nagoya University have observed an interesting phenomenon when studying red blood cells in microfluidic channels. Instead of flowing along the channel in a smooth motion as expected, some cells bounce back and forth between the channel walls in a pinball-like motion at much slower speed. In addition to these ‘cell pinballs’, there are also cells that move at a similar reduced speed, but don’t hit the channel walls.
This altered behaviour could have detrimental effects on microfluidic devices, caused by non-uniform movement of the cells in the channels. In order to prevent these potential problems, the authors have investigated the cause of this behaviour. They noted that cell pinballs only occur when the saline medium is hypotonic, as this causes the cells to inflate due to intake of water. By attaching microbeads to the cells and using a high speed camera, the motion of the cells were studied in more detail. The pinball cells rotated clockwise as they moved to the left of the channel and anticlockwise as they moved to the right of the channel (relative to the direction of the flow).
This observation, combined with the knowledge that the cells were inflated in the hypotonic solution, led the authors to believe that the pinball-motion was occurring due to both the shape of the red blood cell and contact with the channel walls. 3D images obtained using confocal microscopy showed that the upper and lower surfaces of the cells were flattened, confirming that the cells were in contact with the walls.
By studying the different possible deformations of the inflated red blood cells when subjected to flow, the authors found that the contact line (between the cell and wall) and the centre line of the cell were not the same. This explains both types of unexpected cell motion – if the contact line is downstream of the centre line, the cell is unstable to rotational motion and this causes it to move at an angle to the flow, leading to the pinball cells, whereas if the contact line is upstream of the centre line the cell is stable to rotational motion and no displacement occurs, leading to the slow moving non-pinball cells.
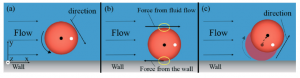
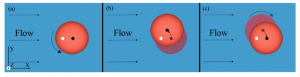
From these studies, the authors were able to propose mechanisms that successfully explained the two types of altered red blood cell behaviour in hypotonic solutions, and hopefully in the future this should allow microfluidic systems to be used which will avoid this pinball-motion occurring.
To download the full article for free* click the link below:
Cell pinball: phenomenon and mechanism of inertia-like cell motion in a microfluidic channel
Ryo Murakami, Chia-Hung Dylan Tsai, Makoto Kaneko, Shinya Sakuma and Fumihito Arai
Lab Chip, 2015, 15, 3307-3313
DOI: 10.1039/ c5lc00535c
—————-

About the webwriter
Claire Weston is a PhD student in the Fuchter Group, at Imperial College London. Her work is focused on developing novel photoswitches and photoswitchable inhibitors.
—————-
*Access is free until 06/09/2015 through a registered RSC account.
An international workshop on microsystems technologies for African health
 µ-Med A 2015, an international workshop on microsystems technologies for African health. This interesting workshop will be held during 16-19 September 2015 at Protea hotel, Stellenbosch, South Africa.
µ-Med A 2015, an international workshop on microsystems technologies for African health. This interesting workshop will be held during 16-19 September 2015 at Protea hotel, Stellenbosch, South Africa.
The event will bring together researchers, technologists, entrepreneurs, non-governmental organizations and funding bodies to interact on the latest developments and future trends in the multidisciplinary field of microsystems technology.
The workshop will focus on the following themes:
Feel free to read more about the success of the first workshop held in 2011.
Register now and contribute to the efforts to improve health in Africa!
For additional information, please visit µ-Med A website.
3D technology has revolutionised the entertainment industry by offering viewers the experience of being part of the action, going on in a movie rather than simply watching it. Thanks to 3D technology, we can sky walk hand in hand with George Clooney in ‘Gravity’.
The history of 3D technology can be drawn all the way back to the invention of the stereoscope by David Brewster in 1844. Last two decades have seen 3D technology replacing 2D in all walks of life, ranging from entertainment, physics, microelectronics, tissue engineering and regenerative medicine. For e.g., microelectromechanosensors (MEMS) are 3D devices produced by using soft lithography techniques. MEMS installed in air-bags in the cars have saved thousands of lives by sensing pressure levels during accidents.
Can we use 3D technology to have a better look at the complex events happening at cellular level? One of the major challenges in tissue engineering is that the conventional approaches are mainly limited to 2D monolayers systems and do not allow manipulation of complex multilayer tissue. Cells grown on 2D substrates may respond and differentiate distinctly than those in more physiologically relevant 3D environments. The emergence of 3D technology has enabled scientists to mimic the exact cellular environments and helped to provide better insights into the cell signalling, migration and differentiation in cells.
One of the ways of mimicking the cellular architectures is bio-etching which involves subtractive manufacturing. Bioetching of monolayers of cells in response to laser cuts or scratch assays is achieved by using 2D cell culture studies. But the actual biological systems such as tissues and organs are much more complex and cannot be mimicked using simple monolayers. For long time, scientists have been working on developing better technologies to address this problem. One of the ways to achieve this is 3D bio-etching.
William C. Messner et al. from Tufts University in a recent article in Lab on a Chip explain the utility of 3D bioetching technique to create and shape 3D composite tissues using a microfluidics based approach. The ability to shape the 3D form of multicellular tissues and to control 3D stimulation will have a high impact on tissue engineering and regeneration applications in bioengineering and medicine as well as provide significant improvements of highly complex 3D integrated multicellular biosystems.
Can 3D bio-etching help us to design tissue architecture of our choice mimicking different biological events? Find out by reading the full paper for free* using the link below:
3D bio-etching of a complex composite-like embryonic tissue
Melis Hazar, Yong Tae Kim, Jiho Song, Philip R. LeDuc, Lance A. Davidson and William C. Messner
Lab Chip, 2015, Advance Article
DOI: 10.1039/C5LC00530B
*Access is free through a registered RSC account.