Hybrid opto-electric manipulation in microfluidics—opportunities and challenges
Aloke Kumar, Stuart J. Williams, Han-Sheng Chuang, Nicolas G. Green and Steven T. Wereley
Lab Chip, 2011, 11, 2135-2148
DOI: 10.1039/C1LC20208A
The equally eye-catching image on the inside front cover is from Valérie Taly and Andrew D. Griffiths (ISIS, Strasbourg) et al., accompanying work on a droplet-based microfluidics method for digital PCR and a method for multiplexing quantitative digital PCR beyond the conventional limitations of color-encoded probes.

Deniz Pekin, Yousr Skhiri, Jean-Christophe Baret, Delphine Le Corre, Linas Mazutis, Chaouki Ben Salem, Florian Millot, Abdeslam El Harrak, J. Brian Hutchison, Jonathan W. Larson, Darren R. Link, Pierre Laurent-Puig, Andrew D. Griffiths and Valérie Taly
Lab Chip, 2011, 11, 2156-2166
DOI: 10.1039/C1LC20128J
Multiplex digital PCR: breaking the one target per color barrier of quantitative PCR
Qun Zhong, Smiti Bhattacharya, Steven Kotsopoulos, Jeff Olson, Valérie Taly, Andrew D. Griffiths, Darren R. Link and Jonathan W. Larson
Lab Chip, 2011, 11, 2167-2174
DOI: 10.1039/C1LC20126C











 This Communication from Nathan B. Crane (University of South Florida) et al. describes new method of droplet transport, combining diode-like conduction and electrowetting on dielectric to achieve continuous electrowetting with a single electrode.
This Communication from Nathan B. Crane (University of South Florida) et al. describes new method of droplet transport, combining diode-like conduction and electrowetting on dielectric to achieve continuous electrowetting with a single electrode.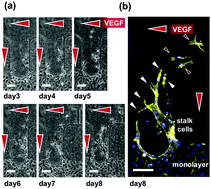 In their paper Seok Chung (Korea University) et al. have developed a hydrogel incorporating a microfluidic platform which can mimic the 3D tissue microenvironment for the study of endothelial cell sprouting angiogenesis. They are able to precisely control the gradient of soluble angiogenic factors, VEGF and ANG-1 and obtain a quantitative response to the assay.
In their paper Seok Chung (Korea University) et al. have developed a hydrogel incorporating a microfluidic platform which can mimic the 3D tissue microenvironment for the study of endothelial cell sprouting angiogenesis. They are able to precisely control the gradient of soluble angiogenic factors, VEGF and ANG-1 and obtain a quantitative response to the assay.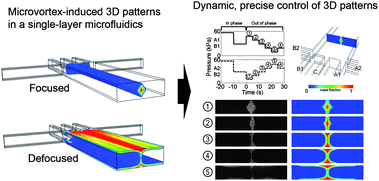 William C. Messner (Carnegie Mellon University) and colleagues have also been working in 3D to achieve dynamic control of 3D chemical patterns in a single 2D microfluidic platform. They are able to switch between ‘focused’ and ‘defocused’ 3D flow profiles, and to rapidly tune the patterns through feedback control of the inlet pressures.
William C. Messner (Carnegie Mellon University) and colleagues have also been working in 3D to achieve dynamic control of 3D chemical patterns in a single 2D microfluidic platform. They are able to switch between ‘focused’ and ‘defocused’ 3D flow profiles, and to rapidly tune the patterns through feedback control of the inlet pressures.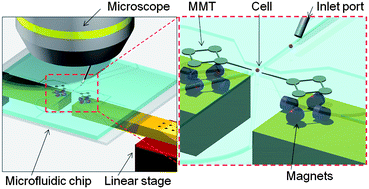 Manipulating cells in microfluidic chips is often accomplished with a magnetically driven microtool (MMT), driven by a permanent magnet. However MMTs driven by permanent magnets suffer from low positioning accuracy and response speed. Here, Masaya Hagiwara (
Manipulating cells in microfluidic chips is often accomplished with a magnetically driven microtool (MMT), driven by a permanent magnet. However MMTs driven by permanent magnets suffer from low positioning accuracy and response speed. Here, Masaya Hagiwara (