Point-of-care diagnostics is the aim for much medical research. Often the problem is the ease of preparation of the biological sample, which usually requires multiple steps before the diagnostic test can even begin. Automation of sample preparation has been explored and another option is pressure-driven microfluidics. However, this requires complex external equipment to run the chip only suitable for use in the laboratory away from the patient.
To get true point-of-care diagnostics, sample preparation has to be fast, simple and cheap.
Stationary microfluidics is now being explored. Liquid is present in fixed positions on the chip and it is magnetic particles that move between the points holding liquid. This often involves separating the liquids using an oil barrier, but this has disadvantages of over-complicating the device by having more than one liquid (the test solution), meaning an additional very careful pipetting step. Not all test solutions or all diagnostic tests would be compatible with this oil barrier, for example, purification of protein. Oil-free versions are now being designed, usually “open droplet” devices, but this again needs accurate pipetting and risks contamination of the sample. Using a large tube limits the miniaturisation capability.
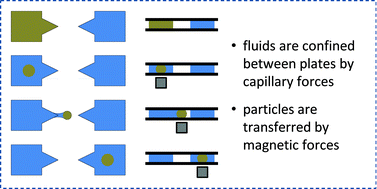 Menno Prins has led a team based at Philips Research and Eindhoven University of Technology, The Netherlands, to solve this problem. They demonstrate technology called Magneto–Capillary Valve (MCV) technology. There is no need for an oil phase. The confinement of the liquid occurs by capillary forces and they can be separated by a gas. The device operation is a balancing act between magnetic and capillary forces.
Menno Prins has led a team based at Philips Research and Eindhoven University of Technology, The Netherlands, to solve this problem. They demonstrate technology called Magneto–Capillary Valve (MCV) technology. There is no need for an oil phase. The confinement of the liquid occurs by capillary forces and they can be separated by a gas. The device operation is a balancing act between magnetic and capillary forces.
In this HOT article, they give numerous device architectures and clearly demonstrate the advantages of this technique in purifying and enriching nucleic acids and proteins. They look in detail at how the device works and its applicability for a wide range of problems in point-of-care-diagnostics.
This research is best read in detail, and as this HOT article is free to access for the next 4 weeks*, you can give it a read now by clicking on the link below:
Magneto-capillary valve for integrated purification and enrichment of nucleic acids and proteins
Remco C. den Dulk, Kristiane A. Schmidt, Gwénola Sabatté, Susana Liébana and Menno W. J. Prins
DOI: 10.1039/C2LC40929A
*Free access to individuals is provided through an RSC Publishing personal account. Registration is quick, free and simple