View the new videos on the Lab on a Chip YouTube site below:
Single- and two-phase flow in microfluidic porous media analogs based on Voronoi tessellation
View the new videos on the Lab on a Chip YouTube site below:
Single- and two-phase flow in microfluidic porous media analogs based on Voronoi tessellation
View the new videos on the Lab on a Chip YouTube site below:
Planar silicon microrings as wavelength-multiplexed optical traps for storing and sensing particles
Converting steady laminar flow to oscillatory flow through a hydroelasticity approach at microscales
Lateral cavity acoustic transducer as an on-chip cell/particle microfluidic switch
Malaysian scientists have created a flexible and environmentally friendly microfluidic device using a cloth decorating technique for printing wax onto cotton.
Dedy Wicaksono at the University of Technology Malaysia was inspired by his batik-patterned clothes to create the device. ‘Batik processing is wax patterning to create regions of differing hydrophilicity and hydrophobicity on cloth,’ says Dedy. The technique is traditionally used to prevent dye spreading from one area of cloth to another, creating coloured patterns. Using wax printing methods for paper and silk based microfluidic devices is known, but these have required specialist equipment or expensive materials.
Wicaksono’s method needs neither of these things. First, his team prepared the cotton by scouring it with sodium hydroxide and anhydrous sodium carbonate solutions. The treatment removes the outer layer, exposing underlying cellulose fibres. Both the chemical composition (increased oxygen content) and physical structure (increased surface roughness) of the fibre surface were altered, increasing the wettability and wicking rate.
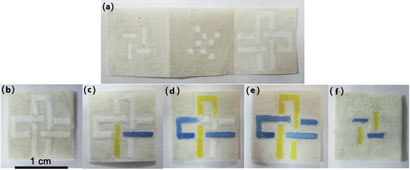
(a)-(f) are photographs showing two hydrophilic channels with different dye solutions that cross each other vertically and horizontally in three layers without mixing. (b)-(e) are front views of the device before, 5 seconds, 2 minutes and 5 minutes after dropping the dyes into different channels. (f) is the bottom layer 5 minutes after adding the dyes.
Then, they printed the pattern for the microfluidic device onto paper. The paper was dipped into hot batik wax, then dried, before the pattern was cut from the sheet and attached to the scoured cloth using a few pins. Heat treatment melted the wax again, and it spread onto the surface and into the cloth, filling the gaps in the weave and within the fibres. The fatty acids in the wax increase the hydrophobicity of the fibres where they are applied, creating barriers to liquid flow.
Dedy’s group made both 2D and 3D devices; the latter was made by folding layers of the patterned cloth. A test using ink solutions revealed the ink moving along the cloth’s hydrophilic channels, filling a device in minutes. In a further test, the team was able to detect the protein bovine serum albumin colorimetrically using the devices, with the result visible to the naked eye.
‘A key innovation here is the batik-inspired method of transferring patterned wax on paper to cotton cloth,’ comments Shashi Murthy, an expert in microfluidic devices at Northeastern University, US. He adds that the technology ‘has the potential to provide a rapid and low cost readout for analytes characterised by relatively simple colorimetric assays’.
Dedy is now investigating ways to exert more control over the liquid flow so that more complex microfluidic devices can be developed. ‘By making the channels inside a flexible cloth, we are envisioning an embeddable wearable lab in the very near future,’ he says.
Flexible microfluidic cloth-based analytical devices using a low-cost wax patterning technique
Azadeh Nilghaz, Dedy H. B. Wicaksono, Dwi Gustiono, Fadzilah Adibah Abdul Majid, Eko Supriyanto and Mohammed Rafiq Abdul Kadir
Lab Chip, 2012, Advance Article
DOI: 10.1039/C1LC20764D
Original article published at Chemistry World
View the new videos on the Lab on a Chip YouTube site below:
On-chip CO2 control for microfluidic cell culture
On-chip measurements of cell compressibility via acoustic radiation
A microfluidic device for self-synchronised production of droplets
A scalable microfluidic chip for bacterial suspension culture
This month sees the following articles in Lab on a Chip that are in the top ten most accessed:
Integration of cell phone imaging with microchip ELISA to detect ovarian cancer HE4 biomarker in urine at the point-of-care
ShuQi Wang, Xiaohu Zhao, Imran Khimji, Ragip Akbas, Weiliang Qiu, Dale Edwards, Daniel W. Cramer, Bin Ye and Utkan Demirci
Lab Chip, 2011, 11, 3411-3418
DOI: 10.1039/C1LC20479C
Paper on a disc: balancing the capillary-driven flow with a centrifugal force
Hyundoo Hwang, Seung-Hoon Kim, Tae-Hyeong Kim, Je-Kyun Park and Yoon-Kyoung Cho
Lab Chip, 2011, Advance Article
DOI: 10.1039/C1LC20445A
Microfluidics with aqueous two-phase systems
Steffen Hardt and Thomas Hahn
Lab Chip, 2011, Advance Article
DOI: 10.1039/C1LC20569B
Large-scale plasmonic microarrays for label-free high-throughput screening
Tsung-Yao Chang, Min Huang, Ahmet Ali Yanik, Hsin-Yu Tsai, Peng Shi, Serap Aksu, Mehmet Fatih Yanik and Hatice Altug
Lab Chip, 2011, 11, 3596-3602
DOI: 10.1039/C1LC20475K
Education: A modular approach to microfluidics in the teaching laboratory
Yolanda Fintschenko
Lab Chip, 2011, 11, 3394-3400
DOI: 10.1039/C1LC90069B
Automated high-throughput generation of droplets
Jan Guzowski, Piotr M. Korczyk, Slawomir Jakiela and Piotr Garstecki
Lab Chip, 2011, 11, 3593-3595
DOI: 10.1039/C1LC20595A
Rounded multi-level microchannels with orifices made in one exposure enable aqueous two-phase system droplet microfluidics
David Lai, John P. Frampton, Hari Sriram and Shuichi Takayama
Lab Chip, 2011, 11, 3551-3554
DOI: 10.1039/C1LC20560A
Microchip-based immunomagnetic detection of circulating tumor cells
Kazunori Hoshino, Yu-Yen Huang, Nancy Lane, Michael Huebschman, Jonathan W. Uhr, Eugene P. Frenkel and Xiaojing Zhang
Lab Chip, 2011, Advance Article
DOI: 10.1039/C1LC20270G
A digital microfluidic method for dried blood spot analysisMais J. Jebrail, Hao Yang, Jared M. Mudrik, Nelson M. Lafrenière, Christine McRoberts, Osama Y. Al-Dirbashi, Lawrence Fisher, Pranesh Chakraborty and Aaron R. Wheeler
Lab Chip, 2011, 11, 3218-3224
DOI: 10.1039/C1LC20524B
Green microfluidic devices made of corn proteins
Jarupat Luecha, Austin Hsiao, Serena Brodsky, Gang Logan Liu and Jozef L. Kokini
Lab Chip, 2011, 11, 3419-3425
DOI: 10.1039/C1LC20726A
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.
View the new videos on the Lab on a Chip YouTube site using the links below:
1-Million droplet array with wide-field fluorescence imaging for digital PCR
Lateral dielectrophoretic microseparators to measure the size distribution of blood cells
Guiding, distribution, and storage of trains of shape-dependent droplets
This month sees the following articles in Lab on a Chip that are in the top ten most accessed:
Automated cellular sample preparation using a Centrifuge-on-a-Chip
Albert J. Mach, Jae Hyun Kim, Armin Arshi, Soojung Claire Hur and Dino Di Carlo
Lab Chip, 2011, 11, 2827-2834
DOI: 10.1039/C1LC20330D
Benchtop micromolding of polystyrene by soft lithography
Yuli Wang, Joseph Balowski, Colleen Phillips, Ryan Phillips, Christopher E. Sims and Nancy L. Allbritton
Lab Chip, 2011, 11, 3089-3097
DOI: 10.1039/C1LC20281B
Next-generation integrated microfluidic circuits
Bobak Mosadegh, Tommaso Bersano-Begey, Joong Yull Park, Mark A. Burns and Shuichi Takayama
Lab Chip, 2011, 11, 2813-2818
DOI: 10.1039/C1LC20387H
Beyond PDMS: off-stoichiometry thiol–ene (OSTE) based soft lithography for rapid prototyping of microfluidic devices
Carl Fredrik Carlborg, Tommy Haraldsson, Kim Öberg, Michael Malkoch and Wouter van der Wijngaart
Lab Chip, 2011, 11, 3136-3147
DOI: 10.1039/C1LC20388F
A high-performance microsystem for isolating circulating tumor cells
Xiangjun Zheng, Luthur Siu-Lun Cheung, Joyce A. Schroeder, Linan Jiang and Yitshak Zohar
Lab Chip, 2011, 11, 3269-3276
DOI: 10.1039/C1LC20331B
Paper on a disc: balancing the capillary-driven flow with a centrifugal force
Hyundoo Hwang, Seung-Hoon Kim, Tae-Hyeong Kim, Je-Kyun Park and Yoon-Kyoung Cho
Lab Chip, 2011, Advance Article
DOI: 10.1039/C1LC20445A
Double-emulsion drops with ultra-thin shells for capsule templates
Shin-Hyun Kim, Jin Woong Kim, Jun-Cheol Cho and David A. Weitz
Lab Chip, 2011, 11, 3162-3166
DOI: 10.1039/C1LC20434C
Rapid spatial and temporal controlled signal delivery over large cell culture areas
Jules J. VanDersarl, Alexander M. Xu and Nicholas A. Melosh
Lab Chip, 2011, 11, 3057-3063
DOI: 10.1039/C1LC20311H
Flexible microfluidic devices with three-dimensional interconnected microporous walls for gas and liquid applications
Po Ki Yuen and Michael E. DeRosa
Lab Chip, 2011, 11, 3249-3255
DOI: 10.1039/C1LC20157C
Microchip-based immunomagnetic detection of circulating tumor cells
Kazunori Hoshino, Yu-Yen Huang, Nancy Lane, Michael Huebschman, Jonathan W. Uhr, Eugene P. Frenkel and Xiaojing Zhang
Lab Chip, 2011, Advance Article
DOI: 10.1039/C1LC20270G
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.
The study of brain development and degeneration is hindered by a lack of physiologically realistic models. French scientists have now developed a way to reconstruct neuronal networks in a microfluidic system to more closely mimic the directional neuronal pathways found in the brain.
Current experimental brain models include neuronal cell cultures and whole animal models; however, the former lack the complex architecture found in vivo, and the latter restrict studies at the cellular level. Aiming to bridge the gap between these models, Jean-Louis Viovy of the Curie Institute, Paris, and coworkers have developed a microfluidic device that allows for the growth of oriented and functional synaptic connections in vitro.
The device consists of two cell culture chambers connected by microchannels, through which axons – nerve fibres that conduct impulses away from the body of the nerve cell – can penetrate to form neuronal networks. In previous setups, replication of the unidirectional networks found in vivo could not be achieved since axons were sent from each chamber to the one opposite, travelling in both directions across the microchannel.
Inspired by the observation that axons can be mechanically constrained, the team modified the device to include asymmetric, funnel-shaped microchannels, termed ‘axon diodes’, to allow axons to grow from only one chamber to the other and not the opposite way. The concept was verified by experiments with mouse cortical neurons, in which the axon projection was 97 per cent selective for the ‘correct’ direction.
![c1lc20014c-image_250_tcm18-207329[1]](https://blogs.rsc.org/lc/files/2011/09/c1lc20014c-image_250_tcm18-20732912.jpg)
Neuronal networks have been grown in microfluidic chambers to replicate neural pathways in the brain
‘I was struck by the simplicity of the system, it is beautiful,’ remarks Bonnie Firestein, an expert in cellular neurobiology from Rutgers University, New Jersey, US. ‘It is very easy to make and to use, and allows the recreation of what happens in vivo in an in vitro system.’
Such a device has many potential applications in neurobiological research. An initial motivation for this work was the requirement of a model to study the progression of neuronal damage in degenerative diseases such as Alzheimer’s. Additionally, Viovy believes the system is also an important platform for research into brain development and cognitive science. ‘How neurons communicate regarding information transmission is also an area in which we currently lack a model of the kind we have proposed here,’ he says. The team are currently working on further increasing the complexity of the networks, to more accurately model the neuronal organisation of the brain.
Interested? Read Sarah Farley’s full Chemistry World article here or download the Lab on a Chip paper:
Axon diodes for the reconstruction of oriented neuronal networks in microfluidic chambers
Jean-Michel Peyrin, Bérangère Deleglise, Laure Saias, Maéva Vignes, Paul Gougis, Sebastien Magnifico, Sandrine Betuing, Mathéa Pietri, Jocelyne Caboche, Peter Vanhoutte, Jean-Louis Viovy and Bernard Brugg
Lab Chip, 2011, Advance Article
DOI: 10.1039/c1lc20014c
View the new videos on the Lab on a Chip YouTube site using the links below:
A switchable digital microfluidic droplet dye-laser
Electrolysis in nanochannels for in situ reagent generation in confined geometries
View the new videos on the Lab on a Chip YouTube site using the links below:
Photoreversible fragmentation of a liquid interface for micro-droplet generation by light actuation
DNA-templated assembly of droplet-derived PEG microtissues
A microchip-based model wound with multiple types of cells
Automated cellular sample preparation using a Centrifuge-on-a-Chip
Double-emulsion drops with ultra-thin shells for capsule templates
Pyroelectric Adaptive Nanodispenser (PYRANA) microrobot for liquid delivery on a target
Microfluidic baker’s transformation device for three-dimensional rapid mixing