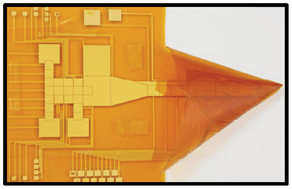
 Lab on a Chip editorial board member Aaron Wheeler and Andrea Kirby at the University of Toronto enable analysis on-chip with a new device format for digital microfluidics (DMF) based chemical synthesis that they are calling “microfluidic origami”.
Lab on a Chip editorial board member Aaron Wheeler and Andrea Kirby at the University of Toronto enable analysis on-chip with a new device format for digital microfluidics (DMF) based chemical synthesis that they are calling “microfluidic origami”.
The setup allows DMF to be integrated with mass spectrometry for in-line analysis. Offline analysis on the benchtop is particularly troublesome for mass spectrometry as it requires further handling steps and takes further time. There are three devices that have coupled DMF with electrospray ionisation before. However, these either have challenges associated with device fabrication or need external equipment and tricky alignment of device and ESI emitter.
This video from the Wheeler laboratory clearly shows how the device is folded, like origami, and explains how it works.
http://www.youtube.com/watch?v=-lpQhPBlu7M
The “origami” aspect is down to a folded nanoelectrospray ionization (nanoESI) emitter on a single flexible polyimide film. This work combines previously known concept of folded ESI emitters and that of making DMF devices with flexible substrates to move droplets on non-planar surfaces to get an integrated device on one flexible substrate. They include a two-plate-to-one-plate DMF interface to give ease of droplet delivery from the mixing region to the nanoESI emitter.
They validate the device with a Morita-Baylis-Hilman reaction, monitoring it in-line in real time. This is the first time a digital microfluidic device has been used to monitor chemical synthesis in real time with in-line mass spectrometry.
This HOT article has been made free to access for the next 4 weeks*, so after watching the video you can read the scientific detail of how they fabricated and validated the device:
Microfluidic origami: a new device format for in-line reaction monitoring by nanoelectrospray ionization mass spectrometry
Andrea E. Kirby and Aaron R. Wheeler
DOI: 10.1039/C3LC41431K