To accurately diagnose a disease or monitor the effectiveness of a treatment, patient samples (e.g. blood, saliva, or urine) must be analyzed in a central laboratory. After collection, the samples will begin to degrade due to chemical, bacterial, and enzymatic interactions.1 These changes can compromise the integrity of the analyte and introduce errors during analysis. Thus, stabilization techniques are necessary to preserve the diagnostic value of patient samples.
Typically, analyte stabilization is achieved by transporting and storing samples at low temperature using dry ice, refrigerators, freezers, or liquid nitrogen. Unfortunately, low-temperature preservation is costly to implement, requiring substantial infrastructure, specialized equipment, and trained personnel. It becomes particularly expensive for applications involving bio surveillance, long-term sample archiving, and clinical diagnostics in low-resource settings.
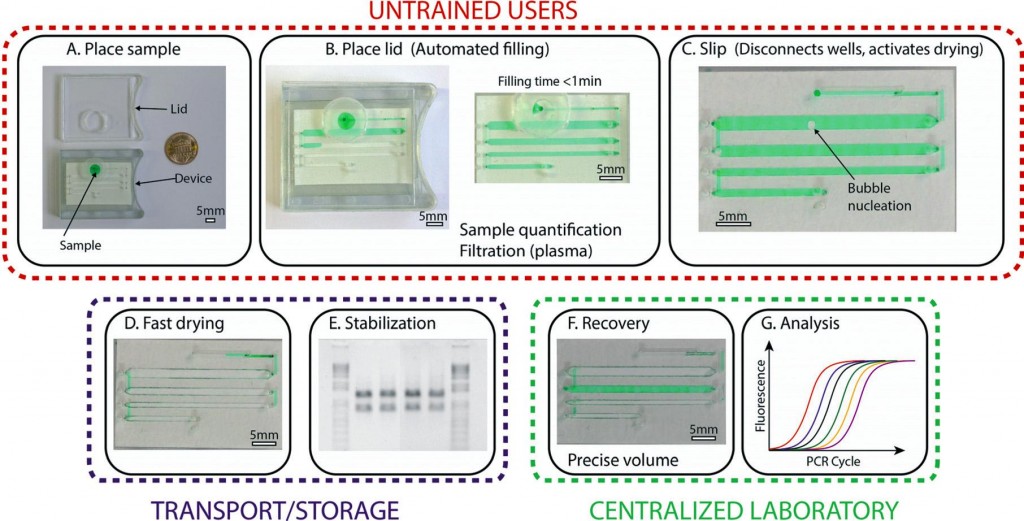
To address this challenge, a research team, led by Prof. Ismagilov at the California Institute of Technology, developed a microfluidic device that can preserve biological samples in the dry state. The device relies on pre-loaded desiccants to rapidly (~30 min) dry samples in a stabilization matrix, protecting the analyte from enzymatic degradation and light or heat activated reactions.2 Importantly, the device is simple to use, allowing minimally trained users to collect and preserve samples without worrying about the precision of the input volume.
The device, based on SlipChip technology,3 was formed by stacking three layers of subassemblies: the top layer has through-holes for sample loading and recovery, the middle layer has channels for sample storage, and the bottom layer houses the desiccants for drying. With the help of a lubricant between each layer, the middle layer can be horizontally moved (slipped), relative to the other layers, allowing the device to be reconfigured into different states.
The device has three states, each with a specific function: loading, drying, and recovery (see figure 1). In the loading state, the loading inlet is connected to the sample storage channel. An untrained user will place the sample in the inlet and close the lid to create a tight seal. This generates an air pressure that pushes the sample through the channels. To initiate the preservation process, the user will slip the device into the drying state. Here, the sample is disconnected from the inlet and is placed into vapor contact with the desiccant through a porous membrane. At this stage, the device can be stored or transported without the use of low-temperature preservation equipment. Just before analysis in the laboratory, a trained user will use a special tool to slip the device into the recovery state. Here, the sample is disconnected from the desiccant chamber and is connected to the recovery inlets. Using a pipette and water, the samples can be rehydrated and recovered for quantitative analysis.
As a validation, the device was subjected to an accelerated aging test (50oC for 5-weeks) while preserving samples containing control RNA or HIV-1 RNA. Remarkably, the RNA samples stored in the device were indistinguishable from the ones stored in the freezer at -80oC, as tested by electrophoresis and RT-qPCR. In contrast, significant degradation was observed in samples stored in the liquid state at 50oC. These results suggest that the RNA may be stable at room temperature in the device for at least up to 8 months.
In summary, the research team developed and validated a microfluidic device that can preserve biological samples in the dry state, eliminating the need for low-temperature equipment. The device is compact, easy to use, making it compatible for challenging applications such as clinical diagnostics in remote, resource-limited settings. The reduction of cost in transport, sample collection, and storage can open up many new possibilities in health care, diagnostics, and beyond.
To access the full article, click the following link: A microfluidic device for dry sample preservation in remote setting, Stefano Begolo, Feng Shen and Rustem F. Ismagilov.
1. G. V. Iyengar, K. S. Subramanian and J. R. W. Woittiez, Element Analysis of Biological Samples: Principles and Practice, CRC Press, Boca Raton, New York, 1998.
2. E. Wan, M. Akana, J. Pons, J. Chen, S. Musone, P. Y. Kwok and W. Liao, Current Issues in Molecular Biology, 2010, 12, 135-142.
3. W. Du, L. Li, K. P. Nichols and R. F. Ismagilov, Lab on a Chip, 2009, 9, 2286-2292.