Renewable, clean energy is all around us. In fact, the amount of solar and wind energy available for harvest is many times higher than the amount consumed by all of civilization. The single most important counter to solar and wind competing with fossil fuels is that they place us at the mercy of nature’s schedule. As a preferred option, historically we’ve gone to great lengths to find energy sources we can turn on and off at will. With the exception of hydro-electric, these technologies are all fuels.
A chemical fuel (as opposed to a nuclear fuel) stores energy between its atoms as molecular bonds. This energy is released as heat when the atoms are rearranged to combine with oxygen from the air; think of coal, gasoline, hydrogen, or wood pellets. As energy sources, chemical fuels are especially attractive because (1) they put lots of energy in a small place, (2) they’re inexpensive to store, (3) they’re easy to move around, and (4) whenever more power is needed it’s relatively simple to fire up more generators, or else shut excess generators off to save energy for later.
The way we spend energy demands the source have an on/off button, but sources of renewable energy can’t be switched on and off like fuels. If we want wind and solar energy to be as reliable as fuel, we have to store them. Storage is a bigger problem than you might think. If any storage technology were developed enough to handle the daily fluctuations in energy demand, our modern discussion over energy would be extremely different. In fact, if wind and solar were capable of providing energy when we want it, there would be little incentive to use fossil fuels. Proposed methods of storage can be boiled down to roughly 3 categories:
- Electrical: Store renewable energy in giant batteries (or capacitors). When energy is desired, flip a switch.
- Mechanical: Use renewable energy to pump water to a raised reservoir, spin a flywheel, or compress air. When energy is desired, have one of these technologies crank a generator.
- Chemical: Turn renewable energy into fuel. When energy is desired, fire up a fuel-powered generator (or fuel cell).
Traditionally, solar and wind energy have been stored in batteries, but consider the advantages of storing this energy as fuel. To stockpile energy electrically we need lots of batteries; to stockpile energy as fuel we need only barrels and tanks. Compared to batteries, the materials and methods for manufacturing and recycling of barrels and tanks are enormously simpler and cheaper. Also, compared to the practically unlimited refilling capacity of barrels and tanks, batteries can be “refilled” only a few hundred times before they must be recycled. These simple reasons – combined with all the advantages of fuels discussed earlier – have convinced me that THE solution for solar and wind replacing fossil fuels is to use them to synthesize renewable fuel. Stated more clearly, we must use solar and wind energy to convert the products of fuel combustion (carbon dioxide and water) back into fuel.
The reason you hear so much about biotech when talking about sustainable energy is that plants and algae are critical to one particular method of using solar energy to turn carbon dioxide and water into fuel: biofuels. Unlike say, a mushroom, a plant gets its carbon from the air as it performs photosynthesis. This is why a tree doesn’t leave a hole in the ground as it grows, yet a mushroom deteriorates whatever it grows out of. Plants, in fact, are practically the only method of converting atmospheric carbon into anything; thermo- and electro-chemical processes generally require concentrated batches of carbon dioxide to work.
While plant products such as wood are technically fuels, they won’t work in your car without modifying either the fuel or the car. It would be hopelessly impractical to power all cars on wood chips (and people do actually do this; look up “woodgas”). Rather, the study of biofuels generally revolves around converting plants into liquid fuel.
There are two halves to the study of biofuels. One half focuses the agricultural end; figuring out how to make plants produce more of the materials which are easy to convert into fuel and/or how to farm more of these plants sustainably. The other half focus on the conversion end; new techniques of thermo-, electro-, or bio-based conversion that can be used on the plants that are easier to grow and/or don’t directly interfere with food production (this is how nature works; the plant materials that are easy to convert tend to be edible).
While there’s true potential to bring renewable energy to the market using biofuels, it’s worth noting that biofuels offer only about 1-3% efficiency. That is to say, out of all the sunlight that falls on an acre of plants, only a small fraction of that energy will make it into the fuel made from those plants. Being mindful of this, there have been separate efforts to develop thermo- and electro-chemical techniques that use catalysts either with the sun’s heat or solar/wind electricity to produce fuel directly from water or stores of carbon dioxide. These technologies go by many names, but I tend to use “water splitting” (which produces hydrogen) and “carbon-dioxide splitting” (which produces carbon monoxide). Some of these technologies produce a mix of hydrogen and carbon monoxide known as “syngas”. These products may either be used as fuels in their own right, or else as precursors to more traditional fuels such as gasoline, jet fuel, or diesel. (For more information, read up on “synthetic fuel”.)
I hope this has outlined why energy storage is such an important issue and offered some understanding of the methods currently being evaluated and researched. Thanks for reading!
By Robert Coolman
You can read about some of the latest research which is helping to address these issues in the Energy & Environmental Science collection on New energy storage devices for post lithium-ion batteries.
Comments Off on The Storage Problem


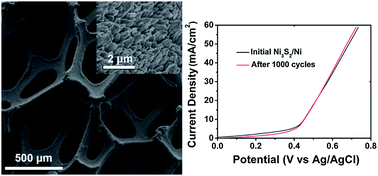
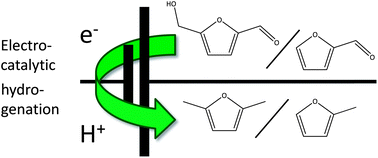
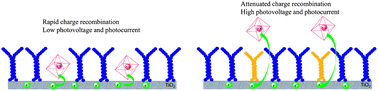











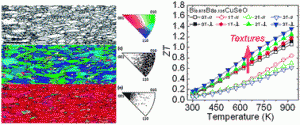
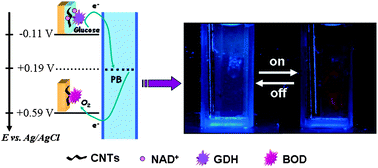


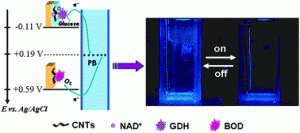
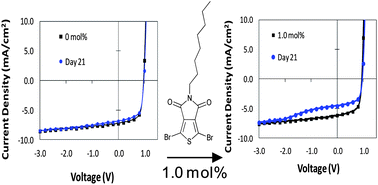
 Take a look at this exciting article that has been recently published online
Take a look at this exciting article that has been recently published online