We are delighted to share with you a series of collections of recent books, themed issues and articles on the topic of water. These four collections – one per month – demonstrate different aspects of water: its chemistry, its wide use in reactions and as a solvent, its relationship with energy and sustainability, as well as with human health and the environment.

Image (c) Shutterstock
Here, in our third collection, we have assembled some of the groundbreaking research and transformative reviews related to water and energy – focussing on the relevance of water in dealing with the challenge of energy production and sustainability – from across our journals.
“Water splitting to generate hydrogen fuel remains one the most challenging scientific and technological problems in renewable energy,” comments Professor Michael Wasielewski, Clare Hamilton Hall Professor of Chemistry at Northwestern University, and Executive Director at the Institute for Sustainability and Energy at Northwestern (ISEN). “This collection of articles highlights a broad array of promising concepts, materials and methods for achieving this goal.”
“This excellent collection of articles highlights more broadly the ongoing interest and high activity around the world in the field of water splitting, solar fuels and the use of water in energy research,” notes Philip Earis, Executive Editor for Energy & Environmental Science, Nanoscale, Physical Chemistry Chemical Physics, Green Chemistry, and Catalysis Science & Technology. “We are delighted to publish impactful science in this important research area across a wide range of Royal Society of Chemistry journals, as the collection nicely illustrates.”
“This year, as the IPCC prepares to release the final contributions to their Fifth Assessment Report on climate change, it is timely to consider the role of chemistry in addressing global challenges, such as food, water, raw materials and energy,” remarks Professor Lesley Yellowlees, President of the Royal Society of Chemistry. “This collection from our journals shares the latest research from scientists around the world, aiming to tackle these challenges. Featuring original research and commentary by leaders in the field, we hope that you will find this high-quality collection engaging, inspirational and informative.”
You can read all of these articles for free until 11 May 2014! We truly hope you enjoy this collection.
We have already published our collections on the Chemistry of water and Chemistry in water. Next month, watch out for our final collection on water in health and the environment.
Did you know that the RSC has put together a webpage on Water, which brings together information on activities for scientists, policymakers, educators and young people? Take a look today…
Related book and themed issue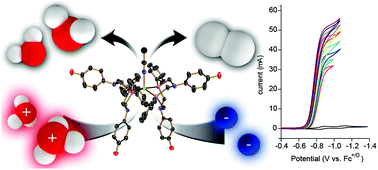
This book and themed issue may be of interest – have a look…
Photoelectrochemical Water Splitting
Editors: Hans-Joachim Lewerenz, Laurie Peter
Copyright: 2013
Print ISBN: 978-1-84973-647-3; PDF eISBN: 978-1-84973-773-9; DOI:10.1039/9781849737739
ChemComm themed issue on Electrocatalytic hydrogen evolution
Reviews and Perspectives
Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: Recent progress and future challenges
Pingwu Du and Richard Eisenberg
Energy Environ. Sci., 2012,5, 6012-6021
DOI: 10.1039/C2EE03250C, Perspective
Plasmonic solar water splitting
Scott C. Warren and Elijah Thimsen
Energy Environ. Sci., 2012,5, 5133-5146
DOI: 10.1039/C1EE02875H, Review Article
Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting
Frank E. Osterloh
Chem. Soc. Rev., 2013,42, 2294-2320
DOI: 10.1039/C2CS35266D, Review Article
From themed collection Solar fuels
Tetrametallic molecular catalysts for photochemical water oxidation
Andrea Sartorel, Marcella Bonchio, Sebastiano Campagna and Franco Scandola
Chem. Soc. Rev., 2013,42, 2262-2280
DOI: 10.1039/C2CS35287G, Review Article
From themed collection Solar fuels
 Nano-architecture and material designs for water splitting photoelectrodes
Nano-architecture and material designs for water splitting photoelectrodes
Hao Ming Chen, Chih Kai Chen, Ru-Shi Liu, Lei Zhang, Jiujun Zhang and David P. Wilkinson
Chem. Soc. Rev., 2012,41, 5654-5671
DOI: 10.1039/C2CS35019J, Tutorial Review
Design and development of photoanodes for water-splitting dye-sensitized photoelectrochemical cells
John R. Swierk and Thomas E. Mallouk
Chem. Soc. Rev., 2013,42, 2357-2387
DOI: 10.1039/C2CS35246J, Review Article
From themed collection Solar fuels
Carbon nanofluidics of rapid water transport for energy applications
Hyung Gyu Park and Yousung Jung
Chem. Soc. Rev., 2014, Advance Article
DOI: 10.1039/C3CS60253B, Tutorial Review
Polyoxometalate water oxidation catalysts and the production of green fuel
Hongjin Lv, Yurii V. Geletii, Chongchao Zhao, James W. Vickers, Guibo Zhu, Zhen Luo, Jie Song, Tianquan Lian, Djamaladdin G. Musaev and Craig L. Hill
Chem. Soc. Rev., 2012,41, 7572-7589
DOI: 10.1039/C2CS35292C, Critical Review
From themed collection Polyoxometalate cluster science
Comparison of primary oxidants for water-oxidation catalysis
Alexander R. Parent, Robert H. Crabtree and Gary W. Brudvig
Chem. Soc. Rev., 2013,42, 2247-2252
DOI: 10.1039/C2CS35225G, Tutorial Review
From themed collection Solar fuels
Earth-abundant hydrogen evolution electrocatalysts
James R. McKone, Smaranda C. Marinescu, Bruce S. Brunschwig, Jay R. Winkler and Harry B. Gray
Chem. Sci., 2014,5, 865
DOI:10.1039/C3SC51711J, Minireview
Charge carrier trapping, recombination and transfer in hematite (α-Fe2O3) water splitting photoanodes
Monica Barroso, Stephanie R. Pendlebury, Alexander J. Cowan and James R. Durrant
Chem. Sci., 2013,4, 2724-2734
DOI: 10.1039/C3SC50496D, Perspective, Open Access
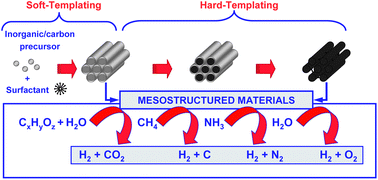 Advances in the design of ordered mesoporous materials for low-carbon catalytic hydrogen production
Advances in the design of ordered mesoporous materials for low-carbon catalytic hydrogen production
David P. Serrano, Juan M. Coronado, Víctor A. de la Peña O’Shea, Patricia Pizarro and Juan Ángel Botas
J. Mater. Chem. A, 2013,1, 12016-12027
DOI: 10.1039/C3TA12453C, Highlight
Solar hydrogen production with semiconductor metal oxides: new directions in experiment and theory
Álvaro Valdés, Jeremie Brillet, Michael Grätzel, Hildur Gudmundsdóttir, Heine A. Hansen, Hannes Jónsson, Peter Klüpfel, Geert-Jan Kroes, Florian Le Formal, Isabela C. Man, Rafael S. Martins, Jens K. Nørskov, Jan Rossmeisl, Kevin Sivula, Aleksandra Vojvodic and Michael Zäch
Phys. Chem. Chem. Phys., 2012,14, 49-70
DOI: 10.1039/C1CP23212F, Perspective
Original research articles
Photocatalytic water gas shift using visible or simulated solar light for the efficient, room-temperature hydrogen generation
Francesc Sastre, Marica Oteri, Avelino Corma and Hermenegildo García
Energy Environ. Sci., 2013,6, 2211-2215
DOI: 10.1039/C3EE40656C, Paper
Modeling, simulation, and design criteria for photoelectrochemical water-splitting systems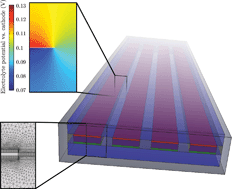
Sophia Haussener, Chengxiang Xiang, Joshua M. Spurgeon, Shane Ardo, Nathan S. Lewis and Adam Z. Weber
Energy Environ. Sci., 2012,5, 9922-9935
DOI: 10.1039/C2EE23187E, Paper
Electrochemical water splitting by layered and 3D cross-linked manganese oxides: correlating structural motifs and catalytic activity
Arno Bergmann, Ivelina Zaharieva, Holger Dau and Peter Strasser
Energy Environ. Sci., 2013,6, 2745-2755
DOI: 10.1039/C3EE41194J, Paper
A hybrid energy cell for self-powered water splitting
Ya Yang, Hulin Zhang, Zong-Hong Lin, Yan Liu, Jun Chen, Ziyin Lin, Yu Sheng Zhou, Ching Ping Wong and Zhong Lin Wang
Energy Environ. Sci., 2013,6, 2429-2434
DOI: 10.1039/C3EE41485J, Communication
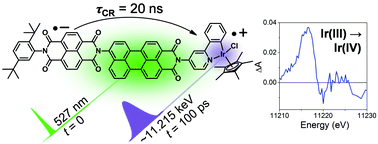 Interrogating the photogenerated Ir(IV) state of a water oxidation catalyst using ultrafast optical and X-ray absorption spectroscopy
Interrogating the photogenerated Ir(IV) state of a water oxidation catalyst using ultrafast optical and X-ray absorption spectroscopy
Michael T. Vagnini, Michael W. Mara, Michael R. Harpham, Jier Huang, Megan L. Shelby, Lin X. Chen and Michael R. Wasielewski
Chem. Sci., 2013,4, 3863-3873
DOI: 10.1039/C3SC51511G, Edge Article
Photocatalytic oxidation of water by polymeric carbon nitride nanohybrids made of sustainable elements
Jinshui Zhang, Marek Grzelczak, Yidong Hou, Kazuhiko Maeda, Kazunari Domen, Xianzhi Fu, Markus Antonietti and Xinchen Wang
Chem. Sci., 2012,3, 443-446
DOI: 10.1039/C1SC00644D, Edge Article
Photocatalytic generation of hydrogen from water using a cobalt pentapyridine complex in combination with molecular and semiconductor nanowire photosensitizers
Yujie Sun, Jianwei Sun, Jeffrey R. Long, Peidong Yang and Christopher J. Chang
Chem. Sci., 2013,4, 118-124
DOI: 10.1039/C2SC21163G, Edge Article
Cu2O|NiOx nanocomposite as an inexpensive photocathode in photoelectrochemical water splitting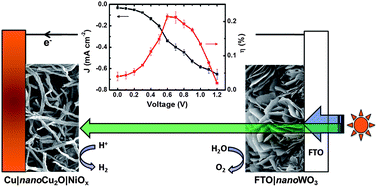
Chia-Yu Lin, Yi-Hsuan Lai, Dirk Mersch and Erwin Reisner
Chem. Sci., 2012,3, 3482-3487
DOI: 10.1039/C2SC20874A, Edge Article
Organic semiconductor for artificial photosynthesis: water splitting into hydrogen by a bioinspired C3N3S3 polymer under visible light irradiation
Zizhong Zhang, Jinlin Long, Lifang Yang, Wenkai Chen, Wenxin Dai, Xianzhi Fu and Xuxu Wang
Chem. Sci., 2011,2, 1826-1830
DOI: 10.1039/C1SC00257K, Edge Article
Passivating surface states on water splitting hematite photoanodes with alumina overlayers
Florian Le Formal, Nicolas Tétreault, Maurin Cornuz, Thomas Moehl, Michael Grätzel and Kevin Sivula
Chem. Sci., 2011,2, 737-743
DOI: 10.1039/C0SC00578A, Edge Article
Cobalt analogs of Ru-based water oxidation catalysts: overcoming thermodynamic instability and kinetic lability to achieve electrocatalytic O2 evolution
Matthew L. Rigsby, Sukanta Mandal, Wonwoo Nam, Lara C. Spencer, Antoni Llobet and Shannon S. Stahl
Chem. Sci., 2012,3, 3058-3062
DOI: 10.1039/C2SC20755A, Edge Article
Mesoporous g-C3N4 nanorods as multifunctional supports of ultrafine metal nanoparticles: hydrogen 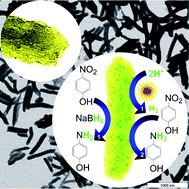 generation from water and reduction of nitrophenol with tandem catalysis in one step
generation from water and reduction of nitrophenol with tandem catalysis in one step
Xin-Hao Li, Xinchen Wang and Markus Antonietti
Chem. Sci., 2012,3, 2170-2174
DOI: 10.1039/C2SC20289A, Edge Article
Photoelectrochemical properties of LaTiO2N electrodes prepared by particle transfer for sunlight-driven water splitting
Tsutomu Minegishi, Naoyuki Nishimura, Jun Kubota and Kazunari Domen
Chem. Sci., 2013,4, 1120-1124
DOI: 10.1039/C2SC21845C, Edge Article
Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water
Daniel Merki, Stéphane Fierro, Heron Vrubel and Xile Hu
Chem. Sci., 2011,2, 1262-1267
DOI: 10.1039/C1SC00117E, Edge Article
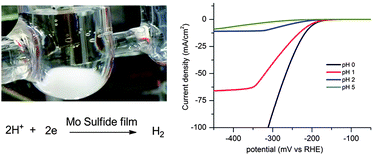
Electrochemical water splitting by gold: evidence for an oxide decomposition mechanism
Oscar Diaz-Morales, Federico Calle-Vallejo, Casper de Munck and Marc T. M. Koper
Chem. Sci., 2013,4, 2334-2343
DOI: 10.1039/C3SC50301A, Edge Article
A light-assisted, polymeric water oxidation catalyst that selectively oxidizes seawater with a low onset potential
Jun Chen, Pawel Wagner, Lei Tong, Danijel Boskovic, Weimin Zhang, David Officer, Gordon G. Wallace and Gerhard F. Swiegers
Chem. Sci., 2013,4, 2797-2803
DOI: 10.1039/C3SC50812A, Edge Article
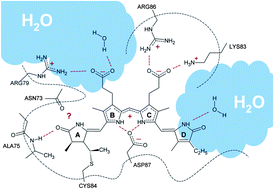 Unraveling the existence of dynamic water channels in light-harvesting proteins: alpha-C-phycocyanobilin in vitro
Unraveling the existence of dynamic water channels in light-harvesting proteins: alpha-C-phycocyanobilin in vitro
Hossam Elgabarty, Peter Schmieder and Daniel Sebastiani
Chem. Sci., 2013,4, 755-763
DOI: 10.1039/C2SC21145A, Edge Article
Wilkinson’s iridium acetate trimer as a water-oxidation catalyst
Alexander R. Parent, James D. Blakemore, Gary W. Brudvig and Robert H. Crabtree
Chem. Commun., 2011,47, 11745-11747
DOI: 10.1039/C1CC15501F, Communication
From themed collection Artificial Photosynthesis
Template-free synthesis of Ta3N5 nanorod arrays for efficient photoelectrochemical water splitting
Chao Zhen, Lianzhou Wang, Gang Liu, Gao Qing (Max) Lu and Hui-Ming Cheng
Chem. Commun., 2013,49, 3019-3021
DOI: 10.1039/C3CC40760H, Communication
Two-step fabrication of a porous γ-In2Se3 tetragonal photocatalyst for water splitting
Ding Wei, Zhengguo Lin, Zhentao Cui, Shuangyue Su, Dingkun Zhang, Minhua Cao and Changwen Hu
Chem. Commun., 2013,49, 9609-9611
DOI: 10.1039/C3CC45598J, Communication
Quantum confinement controlled photocatalytic water splitting by suspended CdSe nanocrystals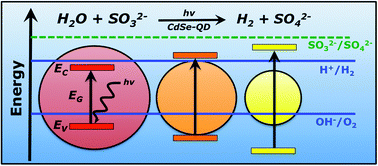
Michael A. Holmes, Troy K. Townsend and Frank E. Osterloh
Chem. Commun., 2012,48, 371-373
DOI: 10.1039/C1CC16082F, Communication
From themed collection Artificial Photosynthesis
Mechanisms for proton release during water oxidation in the S2 to S3 and S3 to S4 transitions in photosystem II
Per E. M. Siegbahn
Phys. Chem. Chem. Phys., 2012,14, 4849-4856
DOI: 10.1039/C2CP00034B, Paper
Synthesis of WO3@Graphene composite for enhanced photocatalytic oxygen evolution from water
Jingjing Guo, Yao Li, Shenmin Zhu, Zhixin Chen, Qinglei Liu, Di Zhang, Won-Jin Moon and Deok-Min Song
RSC Adv., 2012, 2, 1356-1363
DOI: 10.1039/C1RA00621E
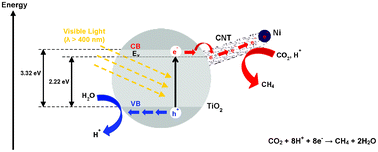 Direct growth of carbon nanotubes on Ni/TiO2 as next generation catalysts for photoreduction of CO2 to methane by water under visible light irradiation
Direct growth of carbon nanotubes on Ni/TiO2 as next generation catalysts for photoreduction of CO2 to methane by water under visible light irradiation
Wee-Jun Ong, Meei Mei Gui, Siang-Piao Chai and Abdul Rahman Mohamed
RSC Adv., 2013, 3, 4505-4509
DOI: 10.1039/C3RA00030C
New spinel oxide catalysts for visible-light-driven water oxidation
Franziska Conrad, Matthias Bauer, Denis Sheptyakov, Stephen Weyeneth, Dominik Jaeger, Kathrin Hametner, Pierre-Emmanuel Car, Jörg Patscheider, Detlef Günther and Greta R. Patzke
RSC Adv., 2012, 2, 3076-3082
DOI: 10.1039/C2RA20169K
Enhanced methane and hydrogen yields from catalytic supercritical water gasification of pine wood sawdust via pre-processing in subcritical water
Jude. A. Onwudili and Paul T. Williams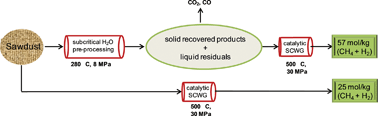
RSC Adv., 2013, 3, 12432-12442
DOI: 10.1039/C3RA41362D
Comments Off on Water and energy














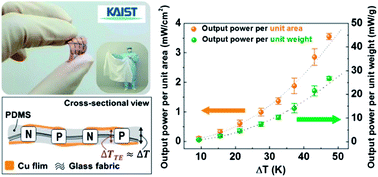
 Researchers in South Korea have devised
Researchers in South Korea have devised 



 Nano-architecture and material designs for water splitting photoelectrodes
Nano-architecture and material designs for water splitting photoelectrodes
 Advances in the design of ordered mesoporous materials for low-carbon catalytic hydrogen production
Advances in the design of ordered mesoporous materials for low-carbon catalytic hydrogen production
 Interrogating the photogenerated Ir(IV) state of a water oxidation catalyst using ultrafast optical and X-ray absorption spectroscopy
Interrogating the photogenerated Ir(IV) state of a water oxidation catalyst using ultrafast optical and X-ray absorption spectroscopy
 generation from water and reduction of nitrophenol with tandem catalysis in one step
generation from water and reduction of nitrophenol with tandem catalysis in one step
 Unraveling the existence of dynamic water channels in light-harvesting proteins: alpha-C-phycocyanobilin in vitro
Unraveling the existence of dynamic water channels in light-harvesting proteins: alpha-C-phycocyanobilin in vitro
 Direct growth of carbon nanotubes on Ni/TiO2 as next generation catalysts for photoreduction of CO2 to methane by water under visible light irradiation
Direct growth of carbon nanotubes on Ni/TiO2 as next generation catalysts for photoreduction of CO2 to methane by water under visible light irradiation 

 systems.
systems. The latest issue of EES is now online. You can read the full issue
The latest issue of EES is now online. You can read the full issue 