Energy & Environmental Science is delighted to present the current issue as a high-profile themed issue on Carbon nanostructures, Guest Edited by Professor Nazario Martin, Professor Dirk M. Guldi and Professor Andreas Hirsch.
 It showcases some of the great current research in this very significant research area, featuring a collection of Perspectives, Minireviews, short Communications and full papers.
It showcases some of the great current research in this very significant research area, featuring a collection of Perspectives, Minireviews, short Communications and full papers.
Minireview
Graphene-based nanomaterials for energy storage
Martin Pumera, Energy Environ. Sci., 2011, 4, 668
Perspective
Underneath the fascinations of carbon nanotubes and graphene nanoribbons
Wei-Tao Zheng and Chang Q Sun, Energy Environ. Sci., 2011, 4, 627
HOT paper
Efficient light harvesting anionic heptamethine cyanine–[60] and [70]fullerene hybrids
Carmen Villegas, Evangelos Krokos, Pierre-Antoine Bouit, Juan Luis Delgado, Dirk M. Guldi and Nazario Martín, Energy Environ. Sci., 2011, 4, 679












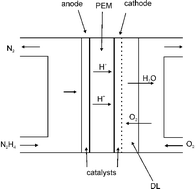 Reviewing recent advances in ammonia and hydrazine based electrochemical fuel cells
Reviewing recent advances in ammonia and hydrazine based electrochemical fuel cells