A remarkable new electrode design represents a significant leap in microbial fuel cell (MFC) technology. Fuel cells using the new cathode have more than two times the power density of current commercially available cathodes.
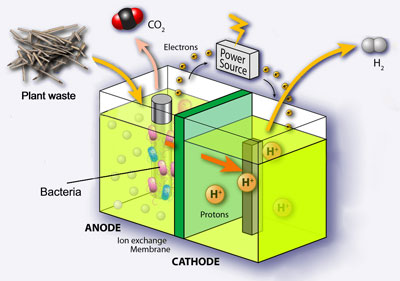
MFCs are a promising technology that is able to act as both a renewable energy source and wastewater treatment method. They use the catalytic activity of microbes to oxidise organic matter in wastewater using oxygen, generating electrical energy. However, fuel cell performance is often limited by the cathode performance.
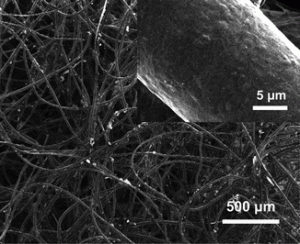
Continuing work previously published, the researchers have developed a new aqueous electrode (i.e. designed to work when submerged in an electrolyte purged with oxygen) in which they deposit platinum nanoparticles onto a carbon nanotube–textile composite.
The electrode allows a maximum MFC power density of 837 mW m−2; using a commercial carbon cloth–Pt electrode only obtains a density of 391 mW m−2.
This enhanced performance is attributed to the cathode’s superior design:
- the network of textile fibres allows a much larger electrochemically active surface area;
- the network also contains macroscale pores for fast access of electrolyte;
- a glacial acetic acid treatment during synthesis provided the electrode with an hydrophilic surface.
Read more about this exciting new work now:
Nano-structured textiles as high-performance aqueous cathodes for microbial fuel cells
Xing Xie, Mauro Pasta, Liangbing Hu, Yuan Yang, James McDonough, Judy Cha, Craig S. Criddle and Yi Cui
Energy Environ. Sci., 2011, Advance Article, DOI: 10.1039/C0EE00793E













 With increasing pressure on non-renewable energy and chemical sources due to the Earth’s swelling population and dwindling supplies, research into renewable and environmentally friendly feedstocks is of critical importance. In this Energy and Environmental Science review, the authors Tadesse and Luque set out the current state of affairs in an area of research which sits at the interface of two important areas of science: ionic liquids and biofuels.
With increasing pressure on non-renewable energy and chemical sources due to the Earth’s swelling population and dwindling supplies, research into renewable and environmentally friendly feedstocks is of critical importance. In this Energy and Environmental Science review, the authors Tadesse and Luque set out the current state of affairs in an area of research which sits at the interface of two important areas of science: ionic liquids and biofuels. Lithium-sulfur batteries are promising rechargeable batteries because of their high energy storage capacity and low cost, but their use has been hindered by their short life cycle and loss of active sulfur through electrochemical reactions in the battery. Porous carbon materials can help as the sulfur is trapped in the pores, preventing it reacting further, but their preparation involves many synthetic steps.
Lithium-sulfur batteries are promising rechargeable batteries because of their high energy storage capacity and low cost, but their use has been hindered by their short life cycle and loss of active sulfur through electrochemical reactions in the battery. Porous carbon materials can help as the sulfur is trapped in the pores, preventing it reacting further, but their preparation involves many synthetic steps.