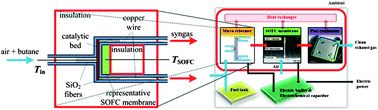
A nanomaterial-based, self-powered sensor that detects mercury in water has been developed by teams from the US and Korea.
Most environmental sensors need to be wired to a power supply, which can be expensive in terms of parts and labour. There is also the potential for contamination from batteries. Solar energy is a more attractive, and greener, alternative, but it relies on weather conditions and time of day.
Instead, Zhong Lin Wang, from the Georgia Institute of Technology, Atlanta, US, and colleagues have made a standalone sensor that harvests energy from movements occurring in its surroundings. They created a nanogenerator to harvest the energy using zinc oxide nanowires (ZnO NW). The nanowires are piezoelectric, which means that they accumulate charge when they are moved, and they’re environmentally friendly.
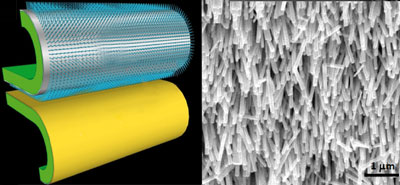
Left: zinc oxide nanowires on a flexible substrate (top) with a gold film electrode (bottom); right: zinc oxide nanowires
The team made the device by placing the nanowires onto a flexible substrate, with the ends of the wires in contact with a gold film electrode. When the nanowires were compressed as a result of movement, electrons flowed along the wires to the gold conductor. With successive compression and release, the electrons flowed back and forth, producing an electrical current. The output was stored in a capacitor to power the sensor to detect pollutants periodically. The sensor was made from single walled carbon nanotubes that turn on an LED indicator. Wang tested the device in water and found that the LED lit up in the presence of mercury ions, and the mercury concentration was indicated by the intensity of the LED.
‘What’s most exciting is that we have built a self-powered system that is driven by energy harvested from the environment that can work independently and sustainably,’ says Wang. In the future, Wang hopes to apply the nanogenerators in other areas besides environmental sensing. ‘There are potential applications in wireless biosensing, sensor networks, personal electronics and even national security,’ he says. His team is also looking at harvesting energy from the environment in other ways such as turbulence in water or air flow and sonic waves.
Jun Liu, from the Pacific Northwest National Laboratory, Richland, US, who works on the synthesis and applications of nanostructured materials for energy was impressed with the device and says that the research has great potential for practical applications. ‘Some biomedical applications or remote area sensing make it difficult to provide the power for very small devices. Fully functional and standalone nanodevices will be handy for these applications,’ he says.
Rebecca Brodie
Read the Energy & Environmental Science article:
Self-powered environmental sensor system driven by nanogenerators
Minbaek Lee, Joonho Bae, Joohyung Lee, Churl-Seung Lee, Seunghun Hong and Zhong Lin Wang
Energy Environ. Sci., 2011, DOI: 10.1039/c1ee01558c











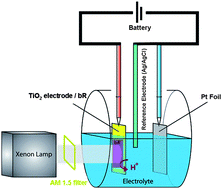 It is thought the proton pumping property of bR can be used in a variety of applications, especially those related to third generation photovoltaic cells.
It is thought the proton pumping property of bR can be used in a variety of applications, especially those related to third generation photovoltaic cells.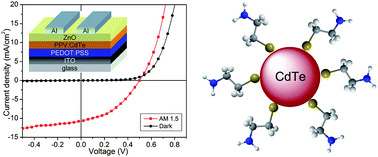
 Energy & Environmental Science is delighted to announce its exciting collaboration with the
Energy & Environmental Science is delighted to announce its exciting collaboration with the