With increasing concerns over global warming and the urgent need to reduce CO2 emissions, scientists in China have developed a new simple and efficient strategy for the reduction of CO2.
They demonstrate a carbon cycle which is driven simply by the oxidation and reduction of commonly available metals, such as iron.
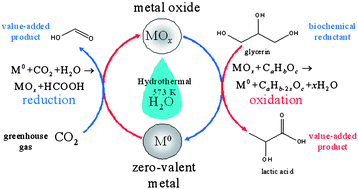
The cycle begins with the high-yield reduction of CO2 to formic, via the oxidation of a zero-valent metal under hydrothermal conditions. The metal oxide can then be converted back to the metal using a bio-derived chemical such as glycerin, which is readily available from renewable resources.
The production of formic acid in the cycle is also an added bonus, as this can be used to power fuel cells, which can be applied to small, portable electronics such as cell phones and laptop computers.
This new energy system has many advantages over current methods to reduce CO2 (such as water-splitting) as it has high yields, no waste products, does not require expensive catalysts or harsh reagents and, as the overall cycle is exothermic, it is expected to have minimal energy requirements.
Read the ‘HOT’ Communication today:
High-yield reduction of carbon dioxide into formic acid by zero-valent metal/metal oxide redox cycles
Fangming Jin, Ying Gao, Yujia Jin, Yalei Zhang, Jianglin Cao, Zhen Wei and Richard L. Smith Jr
Energy Environ. Sci., 2011, DOI: 10.1039/C0EE00661K