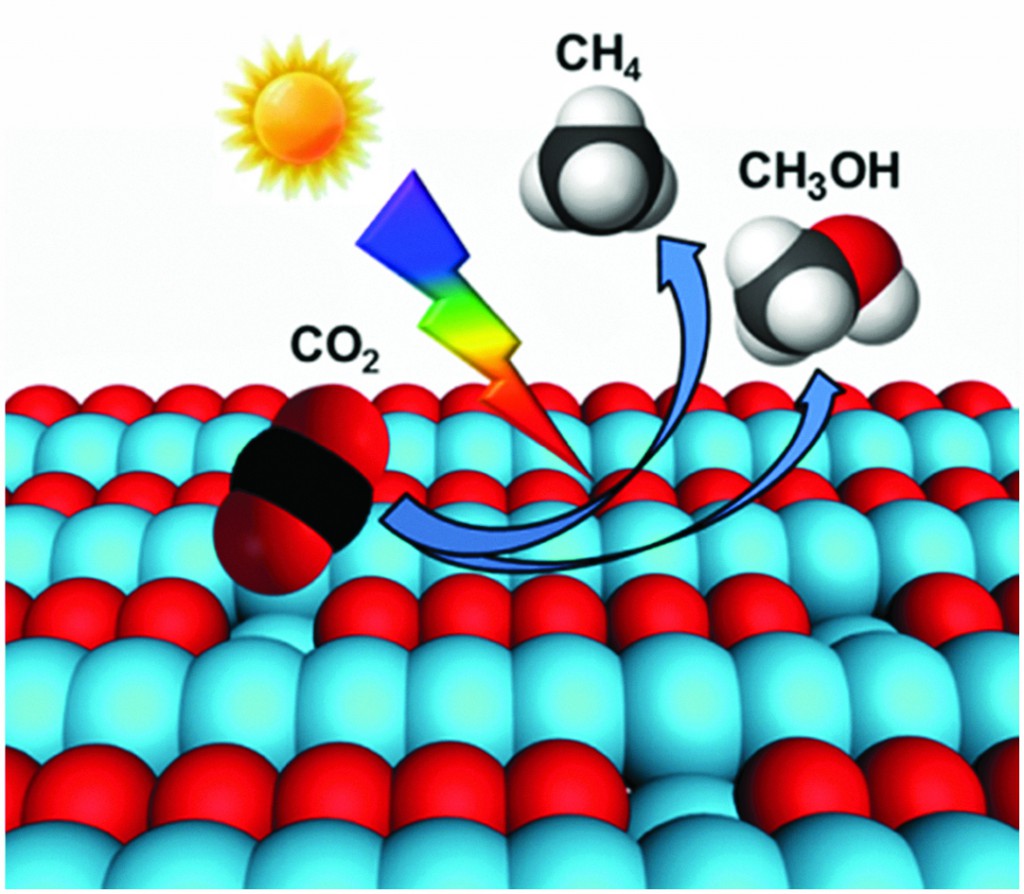
Photocatalytic reduction of CO2 to solar fuels such as methane or methanol may be a promising route to sustainably meet our energy needs. Yet many existing photocatalysts suffer from poor product selectivity and efficiency, caused in part by the competing hydrogen evolution reaction that can occur in the presence of water.
Jinlong Gong and colleagues at Tianjin University, China, highlight recent strategies used to improve the adsorption and activation of CO2 at the catalyst surface – an important and under-researched step in this process – and discuss conditions influencing the reaction pathways leading to the photoreduction products. They also review how chemisorption of the molecule can be enhanced by tailoring properties such as catalyst surface area, surface defects and noble metal co-catalysts, and how factors such as electrolyte pH and CO2 absorption mode can influence product distribution.
Want to know more? Read this review article online, which is free to access until 6 May 2016:
“CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts” by X. Chang et al., DOI: 10.1039/C6EE00383D