| Battery electrodes are typically made from layered oxide materials. However, these layered oxides often undergo a positive ‘strain effect’ or expansion when ions are incorporated into their structure. This can leads to inferior long-term cycling stability and reduced battery safety. However, scientists at the Chinese Academy of Sciences, have synthesised a negative strain layered oxide, Na0.5NbO2, which exhibits high stability, a long cycling life and an impressive rate performance. This material shrinks on intercalation of sodium ions which is thought to be a result of enhanced interlayer Na–O interactions and weakened Nb–Nb and Nb–O bonding. The researchers have also found that the material is suitable as an independent electrode material and as a buffer in composite electrodes, yet the high cost of niobium and the difficulty of synthesis may limit its future application. |
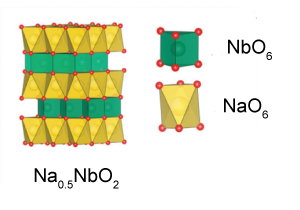 The lattice shrinks upon intercalation of sodium ions The lattice shrinks upon intercalation of sodium ions |
Want to know more?
Read the full article in Chemistry World by Laura Fisher.
Or, take a look at the original article which is free to access until 9th September 2015:
“Anti-P2 structured Na0.5NbO2 and its negative strain effect” by X. Wang et al., DOI:10.1039/C5EE01745A











