 |
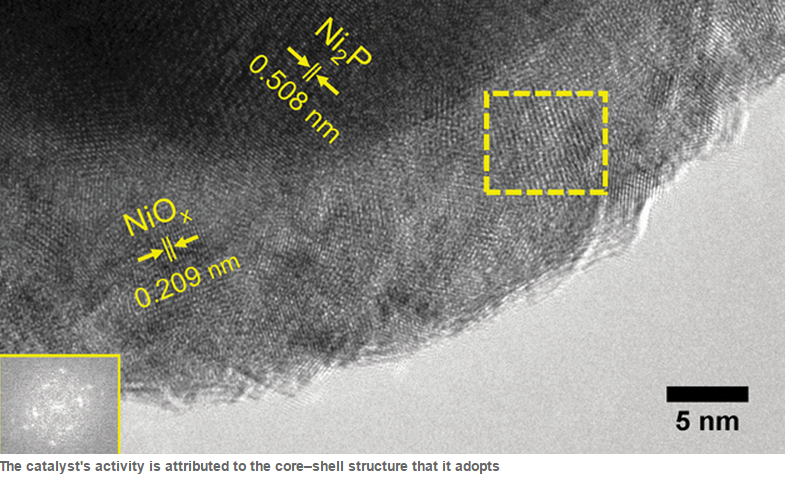
Electrochemical water splitting typically requires two catalysts, one to evolve oxygen and one for hydrogen. However, scientists lead by Xile Hu at EPFL in Lausanne, Switzerland, have discovered that nickel phosphide can act as a catalyst, evolving both hydrogen oxygen from water simultaneously. Nickel phosphide was loaded onto a carbon electrode in an alkaline electrolyser which lead to the material adopting a core-shell structure, with a nickel phosphide core and an active nickel oxide species on the outside. The team observed successful water splitting, with the evolution of both hydrogen and oxygen and a current density of 10mA/cm2 at a low water splitting potential of 1.63V. |
Want to know more?
Read the full article in Chemistry World by Osman Mohamed.
Or, take a look at the original article which is free to access until 7th August 2015:
“Ni2P as a Janus catalyst for water splitting: the oxygen evolution activity of Ni2P nanoparticles” by L-A. Stern et al., DOI: 10.1039/C5EE01155H











