Alexandre Ponrouch and coworkers made significant progress in the optimization of the electrolyte system for sodium-ion batteries. They demonstrate a working sodium-ion battery with an energy density comparable to that of state-of-the-art lithium-ion batteries.
Sodium is both cheaper and more abundant than lithium, and thus sodium-ion batteries are an appealing alternative to lithium-ion batteries for a broad range of energy storage applications. Previous work on Na-ion batteries has demonstrated that hard carbon (HC) electrodes are well suited for the anode of a battery that uses an electrolyte based on a mixture of ethylene carbonate (EC) and propylene carbonate (PC). However, the addition of a third co-solvent can decrease electrolyte viscosity and thus increase its ionic conductivity. Dimethyl carbonate (DMC) was found to be a viable third co-solvent, combining decreased viscosity, increased ionic conductivity and a stable solid electrolyte interphase (SEI) layer (as demonstrated by XPS).
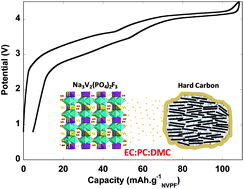
A full battery, utilizing a hard carbon anode, a Na3V2(PO4)2F3 (NVPF) cathode, and an EC/PC/DMC electrolyte was assembled. The battery had an operation voltage of 3.65 V, 110 mA h g-1 specific capacity for the cathode and 300 mA h g-1 specific capacity for the anode. This yields an overall specific energy of 78 Wh kg-1, a figure that compares favorably with that of current Li-ion batteries.
For more information on this exciting advancement in Na-ion batteries, read the full article here:
Towards high energy density sodium ion batteries through electrolyte optimization
Alexandre Ponrouch, Remi Dedryvere, Damien Monti, Atif E. Demet, Jean Marcel Ateba Mba, Laurence Croguennec, Christian Masquelier, Patrik Johansson and M. Rosa Palacin
DOI: 10.1039/c3ee41379a