Yang Shao-Horn, from MIT, and her co-workers report a novel and interesting study of oxygen reduction reaction (ORR) electrocatalysis on epitaxial perovskite thin films of different Mn valance states.
Their findings are very important for the future design of nanostructured catalysts for electrochemical conversion and storage.
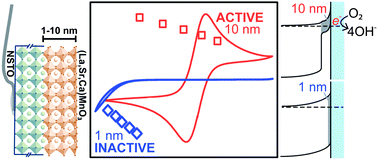
Alkaline fuel cells and metal–air batteries are promising carbon-neutral energy sources. However, these devices suffer from efficiency loss due to the slow ORR kinetics, and the cost of precious metal catalysts required to catalyze the ORR. LaMnO3-based oxides have previously been found to be among the most active for the ORR with activities comparable to that of Pt. However, ambiguity has existed until now as to which Mn valence state on the surface is responsible for the high ORR activity.
Read this HOT article today:
Oxygen electrocatalysis on (001)-oriented manganese perovskite films: Mn valency and charge transfer at the nanoscale
Kelsey A. Stoerzinger, Marcel Risch, Jin Suntivich, W. M. Lü, Jigang Zhou, Michael D. Biegalski, Hans M. Christen, Ariando, T. Venkatesan and Yang Shao-Horn
DOI: 10.1039/C3EE40321A










