Take a look at HOT articles for March. These are only free to acess for 4 weeks only and are available for viewing in a collection on our website.
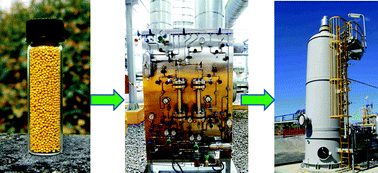
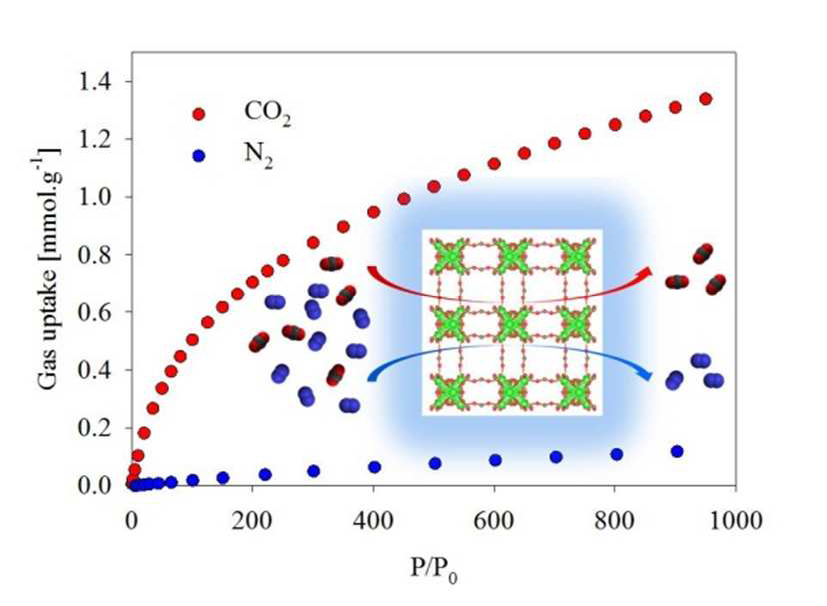
An ionic liquid process for mercury removal from natural gas
Mahpuzah Abai, Martin P. Atkins, Amiruddin Hassan, John D. Holbrey, Yongcheun Kuah, Peter Nockemann, Alexander A. Oliferenko, Natalia V. Plechkova, Syamzari Rafeen, Adam A. Rahman, Rafin Ramli, Shahidah M. Shariff, Kenneth R. Seddon, Geetha Srinivasan and Yiran Zou
Dalton Trans., 2015, Advance Article
DOI: 10.1039/C4DT03273J
Free to access until 24th April 2015
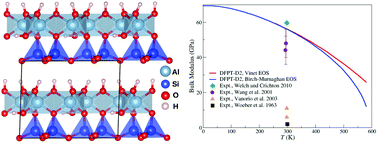
Relationship between crystal structure and thermo-mechanical properties of kaolinite clay: beyond standard density functional theory
Philippe F. Weck, Eunja Kim and Carlos F. Jové-Colón
Dalton Trans., 2015, Advance Article
DOI: 10.1039/C5DT00590F
Free to access until 23rd April 2015
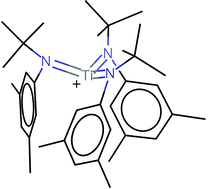
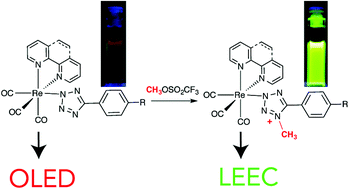
The titanium tris-anilide cation [Ti(N[tBu]Ar)3]+ stabilized as its perfluoro-tetra-phenylborate salt: structural characterization and synthesis in connection with redox activity of 4,4′-bipyridine dititanium complexes
Heather A. Spinney, Christopher R. Clough and Christopher C. Cummins
Dalton Trans., 2015, Advance Article
DOI: 10.1039/C5DT00105F
Free to access until 14th April 2015
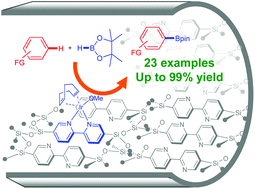
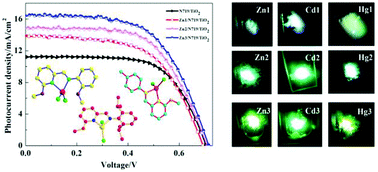
Iridium–bipyridine periodic mesoporous organosilica catalyzed direct C–H borylation using a pinacolborane
Yoshifumi Maegawa and Shinji Inagaki
Dalton Trans., 2015, Advance Article
DOI: 10.1039/C5DT00239G
Free to access until 14th April 2015