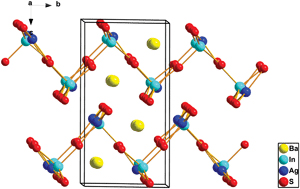
 Multinary chalcogenides have been synthesised for decades, some of these structures have interesting magnetic, thermoelectric and optical properties. Many contain at least one alkali metal while there are relatively few containing alkaline earths, examples of the latter variety include BaM4Se7 (M = Al, Ga) and Ba5Ga4Se10.
Multinary chalcogenides have been synthesised for decades, some of these structures have interesting magnetic, thermoelectric and optical properties. Many contain at least one alkali metal while there are relatively few containing alkaline earths, examples of the latter variety include BaM4Se7 (M = Al, Ga) and Ba5Ga4Se10.
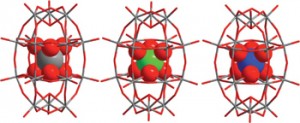
Jiyong Yao et al. describe in their HOT article the synthesis of the first two members of the alkaline-earth/group XI/group XIII/chalcogen system, which is not only an interesting alkaline earth chalcogenide but also the first reported quaternary arrangement. The analogue Ba4LiGa5Se12 was also synthesised and the three new types of structure were fully characterized and the optical properties determined.
To find out more about this Dalton Transactions Hot Article read the full paper which is free to access for 4 weeks.
Ba2AgInS4 and Ba4MGa5Se12 (M = Ag, Li): syntheses, structures, and optical properties
Wenlong Yin, Kai Feng, Dajiang Mei, Jiyong Yao, Peizhen Fu and Yicheng Wu
Dalton Trans., 2012, Advance Article
DOI: 10.1039/C2DT11895E