Posted on behalf of Stuart Bartlett, web writer for Dalton Transactions
Sustainable chemistry is a key area of research, with catalysis leading the way by performing many chemical transformations while only requiring stoichometric amounts. Yet, a problematic area of homogeneous catalysis is high amounts of organic and toxic solvents required, where the catalyst must be separated, and in almost all cases destroyed, in order to recover the product.
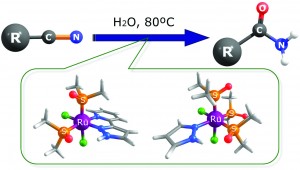
This research, carried out at the University of Girona, in Spain, looked at the hydration of harmful nitriles using a ruthenium based homogeneous catalyst, to its non-toxic amide. This is the first instance of these types of catalysts doing this hydration in solely water or glycerol. This could have a big impact on producing industrially important chemicals much more cheaply and in a sustainable way, while also allowing the removal of toxic chemicals in environmentally friendly media.
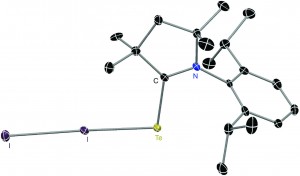
Two catalysts were investigated: [RuCl2(pypz-H)(DMSO)2] (2), and [RuCl2(pz-H)(DMSO)3] (3). (pypz-H = asymmetric didentate 2-(3-pyrazolyl) pyridine ligand; pz-H = monodentate pyrazole ligand; DMSO = dimethyl sulfoxide) Both were tested in H2O at 80oC, at 1 mol % of ruthenium. The substrates included various small organics with a nitrile and another functional group. The conversion of the substrate was above 80% in many cases and selectivity to the corresponding amide, over 98% in almost all cases. In general, catalyst (3) performed better, reasoned as greater flexibility of the catalytic intermediate, having only monodentate ligands. This means more labile sites for the reactants to coordinate. Free DMSO was found after the reaction suggesting these are the labile sites at the metal centre. A further study demonstrated how using glycerol allows recycling of the catalyst with no loss of conversion, where the products go into organic media and the catalyst remains in the glycerol.
This is an excellent study displaying how homogeneous catalysis can move one step further into the field of green chemistry by using non-toxic and plentiful solvents, such as water. Many of these delicate transformations are performed by expensive metals, such as ruthenium, and this work demonstrates how keeping the catalyst in its original media allows for sequential runs, meaning overall lower amounts of the catalyst are required.
Find out more from the paper:
Ru(II) complexes containing dmso and pyrazolyl ligands as catalysts for nitrile hydration in environmentally friendly media
Íngrid Ferrer, Jordi Rich, Xavier Fontrodona, Montserrat Rodríguez and Isabel Romero
Dalton Trans., 2013,42, 13461-13469
DOI: 10.1039/C3DT51580J, Paper
 Stuart Bartlett is currently doing a 1 year postdoc position with David Cole-Hamilton at the University of St Andrews, focusing on the conversion of renewable oils towards fine chemical production using metathesis. He obtained his PhD from the University of Southampton investigating the mechanism of ethene oligomerisation catalysis using NMR and X-ray Absortion Spectroscopy.
Stuart Bartlett is currently doing a 1 year postdoc position with David Cole-Hamilton at the University of St Andrews, focusing on the conversion of renewable oils towards fine chemical production using metathesis. He obtained his PhD from the University of Southampton investigating the mechanism of ethene oligomerisation catalysis using NMR and X-ray Absortion Spectroscopy.













![Water detection by “turn on” fluorescence of the quinone-containing complexes [Ru(phen)2(1,10-phenanthroline-5,6-dione)2+] and [Ru(phenanthroline-5,6-dione)3]2+ Water detection by “turn on” fluorescence of the quinone-containing complexes [Ru(phen)2(1,10-phenanthroline-5,6-dione)2+] and [Ru(phenanthroline-5,6-dione)3]2+](https://blogs.rsc.org/dt/files/2013/08/c3dt51664d-ga-300x250.jpg)


 The conference “
The conference “
![Synthesis, structures and magnetic properties of a family of nitrate-bridged octanuclear [Na2Ln6] (Ln= Dy, Tb, Gd, Sm) complexes Synthesis, structures and magnetic properties of a family of nitrate-bridged octanuclear [Na2Ln6] (Ln= Dy, Tb, Gd, Sm) complexes](https://blogs.rsc.org/dt/files/2013/05/c3dt50417d-ga1-285x300.jpg)