This HOT paper by Ian Dance, from the University of New South Wales, reports on the mechanism of proton relay in nitrogenase enzymes. The proton relay from the protein surface to a key sulfur atom of the FeMo-cofactor plays a core role in many of nitrogenase’s chemical mechanisms.
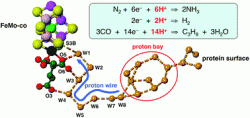
The water chain in nitrogenase is comparable with the purported proton pumping pathway of cytochrome c oxidase.
To read more about the detailed analysis of this mechanism you can download the full paper below…
The controlled relay of multiple protons required at the active site of nitrogenase
Ian Dance
Dalton Trans., 2012
DOI: 10.1039/C2DT30518F
Here are some of Ian Dance’s other recent Dalton Transactions publications:
Ramifications of C-centering rather than N-centering of the active site FeMo-co of the enzyme nitrogenase
Ian Dance
Dalton Trans., 2012,41, 4859-4865
DOI: 10.1039/C2DT00049K, Paper
Calculated vibrational frequencies for FeMo-co, the active site of nitrogenase, bearing hydrogen atoms and carbon monoxide
Ian Dance
Dalton Trans., 2011,40, 6480-6489
DOI: 10.1039/C1DT10505A, Paper
How does vanadium nitrogenase reduce CO to hydrocarbons?
Ian Dance
Dalton Trans., 2011,40, 5516-5527
DOI: 10.1039/C1DT10240K, Paper
Mimicking nitrogenase
Ian Dance
Dalton Trans., 2010,39, 2972-2983
DOI: 10.1039/B922606K, Perspective
From themed issue Bioinspired catalysis
 Are you following us on Twitter? @DaltonTrans
Are you following us on Twitter? @DaltonTrans










