The ability of hydrogenase enzymes to reversibly catalyse the reduction of protons to form molecular hydrogen (H2) has attracted a great deal of research interest. Synthetic mimics of the hydrogenase active site could potentially replace expensive platinum catalysts in hydrogen fuel cells. Currently these mimics are significantly less efficient than the natural enzyme and they are oxygen sensitive. It is thought that by creating a stable peptide based enviroment around the mimic, the stability and efficiency could be improved.
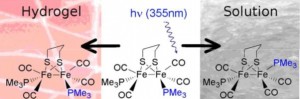
Encapsulating a hydrogenase mimic into a hydrogel causes a restriction in isomerisation after photolysis
Researchers from the Hunt and Ulijn groups at the University of Strathclyde and the Pickett group from the University of East Anglia have successfully encapsulated a [FeFe]-hydrogenase active site mimic into a dipeptide based hydrogelator. Their research has shown that there is a stark difference in the behaviour of the active site mimic in the gel phase compared to the solution phase. Experiments show that the mimic is significantly more stable in the gel phase and is less senstive to water and UV light which could potentially help to improve catalytic activity.
To find out more, read the full Daltons Transactions article…
Encapsulating [FeFe]-hydrogenase model compounds in peptide hydrogels dramatically modifies stability and photochemistry
Pim Wilhelmus, Johannes Maria Frederix, Rafal Kania, Joseph A Wright , Dimitrios A Lamprou, Rein Ulijn, C J Pickett and Neil T Hunt.
This article is part of the upcoming Dalton Discussion themed issue on Inorganic Photophysics and Photochemistry – Fundamentals and Applications










