When designing new chemical sensors and switches, it is desirable to synthesise materials that are able to retain their structural features even after the uptake of a small molecule. Usually to achieve this, polymeric materials are used with extensive strong bonding that are able to withstand structural changes. 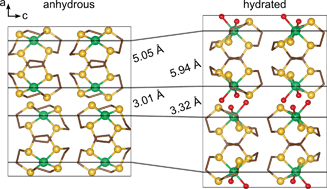
Wojciech Grochala and his team began investigating macrocyclic nickel complexes and discovered a complex that could absorb, then desorb water with retention of its crystallinity. The molecular crystal consists of an almost square planar coordination of nickel(II) supported by a 10 membered, tetradentate sulfur macrocycle. During their investigations, the team discovered that the uptake and release of water vapour was reversible and was accompanied by a change in colour and magnetic susceptibility. The flexible nature of the macrocyclic ligand allows for Ni(12aneS4)(BF4)2 to keep its crystallinity upon water uptake which is rare for molecular crystals and a stark contrast to previously studied coordination polymers.
This exciting finding opens up the possibility of nickel molecular crystals being used as moisture sensors in the future.
To find out more, download the full Dalton Transactions paper:
Chemo-switched chromatic, magnetic and structural changes with retention of molecular crystallinity, Ni(12aneS4)(BF4)2
Andrew J. Churchard, Mariana Derzsi, Zvonko Jagličić, Arndt Remhof and Wojciech Grochala










