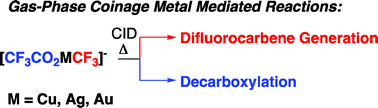
 The presence of C–F bonds in organic compounds has a dramatic influence on their physical, chemical and biological properties. Metal mediated reactions that result in C–F bond formation or C–F bond activation have attracted considerable attention as the demand for organofluorine compounds has increased. Metal-catalysed decarboxylative cross-coupling reactions using trifluoracetate as a cheap source of introducing “CF3” represents an attractive approach. Until now, little has been known about the fragmentation mechanisms of metal trifluoracetates. In this Dalton Transactions HOT article, Rijs and O’Hair use a combination of gas-phase 3D quadrupole ion trap mass spectrometry experiments and density functional theory (DFT) calculations to examine the mechanism of thermal decomposition of fluorinated coinage metal carboxylates. Synthetic chemists should be able to use these results to design new ways of incorporating CF3 and F fragments using trifluoroacetate.
The presence of C–F bonds in organic compounds has a dramatic influence on their physical, chemical and biological properties. Metal mediated reactions that result in C–F bond formation or C–F bond activation have attracted considerable attention as the demand for organofluorine compounds has increased. Metal-catalysed decarboxylative cross-coupling reactions using trifluoracetate as a cheap source of introducing “CF3” represents an attractive approach. Until now, little has been known about the fragmentation mechanisms of metal trifluoracetates. In this Dalton Transactions HOT article, Rijs and O’Hair use a combination of gas-phase 3D quadrupole ion trap mass spectrometry experiments and density functional theory (DFT) calculations to examine the mechanism of thermal decomposition of fluorinated coinage metal carboxylates. Synthetic chemists should be able to use these results to design new ways of incorporating CF3 and F fragments using trifluoroacetate.
Read more for FREE until the 13th March 2012 at:
Forming trifluoromethylmetallates: competition between decarboxylation and C–F bond activation of group 11 trifluoroacetate complexes, [CF3CO2ML]–
Nicole J. Rijs and Richard A. J. O’Hair
Dalton Trans., 2012, Advance Article
DOI: 10.1039/C2DT12117D