Metal Organic Frameworks (MOFs) have received a great deal of attention over the past fifteen years and have proved applicable in a variety of areas including gas storage, catalysis, gas purification and drug delivery. For catalysis, MOFs have been reported that can catalyze a range of organic transformations. By modifying the internal structure of MOFs that allow them to interact with guest species, their chemical reactivity can be fine tuned.
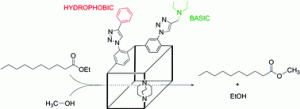
In this article Marie Savonnet et al. have post synthetically modified MOFs by using “click chemistry” for application in base catalysis. Basic catalyltic amino centres and lipophillic phenyl groups were successfully introduced into the DMOF parent structure. These new functionalized MOFs are able to catalyse the transesterification of ethyldecanoate in methanol with good performance. The substrates were co-adsorbed into the MOF structure and from the presence of basic amino groups in the internal MOF structure, methanol can be activated for nucleophillic attack of the ethyldecanoate carbonyl group. This paper demonstrates that through rational design, MOF catalysts can be engineered with appropriate pore size and functionalities for a particular application.
To find out more about this Dalton Transactions Hot Article read the full paper
Tailoring metal-organic framework catalysts by click chemistry
Marie Savonnet, Aurélie Camarata, Jerome Canivet, Delphine Bazer-Bachi, Nicolas Bats, Vincent Lecocq, Catherine Pinel and David Farrusseng
Dalton Trans., 2012, Advance Article
DOI: 10.1039/C2DT11994C