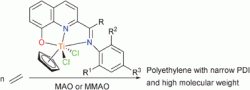
Synthetic polymers are everywhere in modern life and offer multiple opportunities for future materials with a wide range of applications. Using transition metal complex catalysts to precisely control olefin polymerisation is of current interest as they allow the synthesis of polyolefins with set microstructures. Carl Redshaw, Wen-Hua Sun and colleagues have synthesised and fully characterised a series of half-titanocene dichloride 2-aryliminoquinolin-8-olates and tested them with modified methylaluminoxane co-catalysts in ethylene polymerisations and co-polymerisations.
Download the paper today to find out more, including how bulky substituents at the arylimino group of ligands modified the catalytic activities of the complexes:
Synthesis, characterization, and the ethylene (co-)polymerization behaviour of half-titanocene dichloride 2-aryliminoquinolin-8-olates
Wei Huang, Wenjuan Zhang, Wen-Hua Sun, Lin Wang and Carl Redshaw
Catal. Sci. Technol., 2012
DOI: 10.1039/C2CY20240A, Paper
Why not take a look at some other recent work from the team that we have published in Catalysis Science & Technology:
Nickel bis{4,6-dibenzhydryl-2-[(arylimino)methyl]phenoxylate} complexes: Synthesis, structures, and catalytic behaviour towards ethylene and norbornene
Zihong Zhou, Xiang Hao, Carl Redshaw, Langqiu Chen and Wen-Hua Sun
Catal. Sci. Technol., 2012,2, 1340-1345
DOI: 10.1039/C2CY20028G, Paper
Remember all Catalysis Science & Technology articles are currently free to access… sign up for free access now!
You might also find the 2009 Dalton Transactions themed issue on Metal-Catalysed Polymerisation interesting.













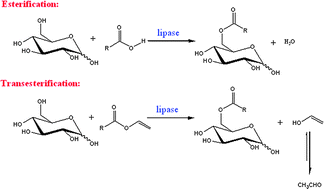 In this Perspective, the use of ionic liquids as alternative reaction media to traditional organic solvents for the biosynthesis of sugar fatty acids esters is explored. Sugar fatty acids are extremely important industrially as non-ionic surfactants in a wide range of applications in food, cosmetics and pharmaceuticals.
In this Perspective, the use of ionic liquids as alternative reaction media to traditional organic solvents for the biosynthesis of sugar fatty acids esters is explored. Sugar fatty acids are extremely important industrially as non-ionic surfactants in a wide range of applications in food, cosmetics and pharmaceuticals.