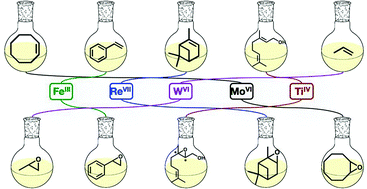
Simone Hauser, Mirza Cokoja and Fritz Kühn explore recent developments in homogeneous epoxidation catalysts in this hot Catalysis Science & Technology Perspective. They look at the different catalysts used for different olefins as well as thinking about the context in which the catalysts would be used.
The manuscript is currently free so download it now, it might help you decide which catalyst to use….
Epoxidation of olefins with homogeneous catalysts – quo vadis?
Simone A. Hauser, Mirza Cokoja and Fritz E. Kühn
Catal. Sci. Technol., 2013
DOI: 10.1039/C2CY20595E
Other Catalysis Science & Technology articles by the same author are:
Xylyltrioxorhenium – the first arylrhenium(VII) oxide applicable as an olefin epoxidation catalyst
Stefan Huber, Mirza Cokoja, Markus Drees, János Mínk and Fritz E. Kühn
Catal. Sci. Technol., 2013
DOI: 10.1039/C2CY20371E, Paper
PtO2 as a “self-dosing” hydrosilylation catalyst
Sophie Putzien, Eckhart Louis, Oskar Nuyken and Fritz E. Kühn
Catal. Sci. Technol., 2012, 2, 725-729
DOI: 10.1039/C2CY00367H
Methyltrioxorhenium-catalysed oxidation of pseudocumene in the presence of amphiphiles for the synthesis of vitamin E
Mónica Carril, Philipp Altmann, Werner Bonrath, Thomas Netscher, Jan Schütz and Fritz E. Kühn
Catal. Sci. Technol., 2012, 2, 722-724
DOI: 10.1039/C1CY00313E