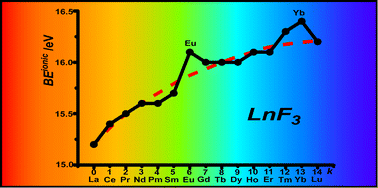
 The complex electronic structure of the lanthanides and actinides is largely a mystery. The f-shell is not readily explained in our theoretical treatment of atoms and molecules, and coupled with the difficulty of obtaining readily interpreted experimental data. The result is that we are still struggling to understand the elements at the bottom of the periodic table. Xu and co-workers conduct a good and meticulous computational study of lanthanide trifluorides, and do significantly increase our understanding of the rare earth elements, albeit in simple molecules in the gas phase. My problem is that I work in aqueous solution, where lanthanide ions have not just three ligands—as the molecules investigated in this paper have—they have eight, nine or ten.
The complex electronic structure of the lanthanides and actinides is largely a mystery. The f-shell is not readily explained in our theoretical treatment of atoms and molecules, and coupled with the difficulty of obtaining readily interpreted experimental data. The result is that we are still struggling to understand the elements at the bottom of the periodic table. Xu and co-workers conduct a good and meticulous computational study of lanthanide trifluorides, and do significantly increase our understanding of the rare earth elements, albeit in simple molecules in the gas phase. My problem is that I work in aqueous solution, where lanthanide ions have not just three ligands—as the molecules investigated in this paper have—they have eight, nine or ten.
The bonding orbitals in the lanthanide series can, in theory, be 4f, 5d, and 6s orbitals. Traditionally, the contracted f-shell orbitals have been viewed as shielded, and the interaction between lanthanide ions and ions of the opposite charge has to be regarded as purely ionic in nature. This work shows that our current computational models include a significant covalent contribution in the bonding of fluoride to trivalent lanthanide ions.
I look forward to the paper where lanthanide ions with a full coordination sphere are treated, although I fear it will be a long wait. Luckily, the papers reporting the progress towards a full computational treatment of lanthanide in solution are also worth reading, even for experimentalists like me.
On structure and bonding of lanthanoid trifluorides LnF3 (Ln = La to Lu)
Wei Xu, Wen-Xin Ji, Yi-Xiang Qiu, W. H. Eugen Schwarz and Shu-Guang Wang
Phys. Chem. Chem. Phys., 2013, 15, 7839-7847
DOI: 10.1039/C3CP50717C
by Dr Thomas Just Sørensen
Comments Off on The mysterious f-block


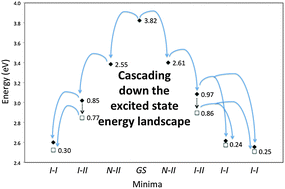









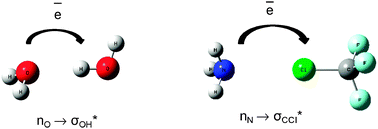
 The complex electronic structure of the lanthanides and actinides is largely a mystery. The f-shell is not readily explained in our theoretical treatment of atoms and molecules, and coupled with the difficulty of obtaining readily interpreted experimental data. The result is that we are still struggling to understand the elements at the bottom of the periodic table. Xu and co-workers conduct a good and meticulous computational study of lanthanide trifluorides, and do significantly increase our understanding of the rare earth elements, albeit in simple molecules in the gas phase. My problem is that I work in aqueous solution, where lanthanide ions have not just three ligands—as the molecules investigated in this paper have—they have eight, nine or ten.
The complex electronic structure of the lanthanides and actinides is largely a mystery. The f-shell is not readily explained in our theoretical treatment of atoms and molecules, and coupled with the difficulty of obtaining readily interpreted experimental data. The result is that we are still struggling to understand the elements at the bottom of the periodic table. Xu and co-workers conduct a good and meticulous computational study of lanthanide trifluorides, and do significantly increase our understanding of the rare earth elements, albeit in simple molecules in the gas phase. My problem is that I work in aqueous solution, where lanthanide ions have not just three ligands—as the molecules investigated in this paper have—they have eight, nine or ten.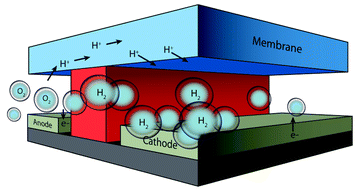 Segalman, Ager and co-authors present a versatile microfluidic test-bed for testing of integrated catalysis and mass transport components for energy conversion via water electrolysis in their recent PCCP Communication.
Segalman, Ager and co-authors present a versatile microfluidic test-bed for testing of integrated catalysis and mass transport components for energy conversion via water electrolysis in their recent PCCP Communication. The
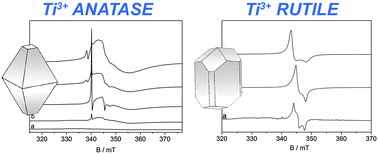
The 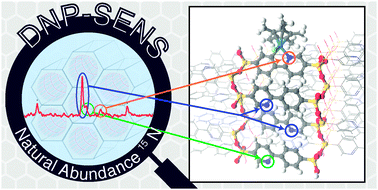 The development of new experimental methods to probe surface functionality is crucial to the understanding of functional materials. For the typically low concentrations of surface functional groups, traditional nuclear magnetic resonance (NMR) spectroscopy lacks the sensitivity to provide chemical information quickly.
The development of new experimental methods to probe surface functionality is crucial to the understanding of functional materials. For the typically low concentrations of surface functional groups, traditional nuclear magnetic resonance (NMR) spectroscopy lacks the sensitivity to provide chemical information quickly.