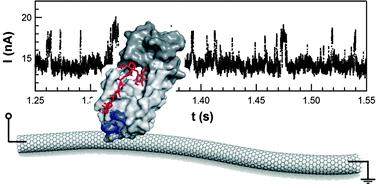
It’s no secret that I find rare gas chemistry very exciting, so when I came across this article by Jien-Lian Chen et al. I knew immediately that it would form the subject of my next blog post. The group, based in Taiwan, have performed calculations that predict a new series of rare gas molecules. Whilst this is, in itself, an exciting achievement, what really grabbed me was that they predict these molecules to be relatively stable – stable enough to be detected experimentally.
As far as I am aware, that is the holy grail of theoretical rare gas chemistry, as there is still very little experimental data on molecules of this kind. The group’s calculations were rigorous, comparing a variety of high-level computational methods and finding good agreement between them. They predicted the strength of the bonds to be significant, and examined the most likely unimolecular dissociation pathways and found sizable barriers to each of them for this class of molecule. All this adds up to a real possibility for generating some new, experimental rare gas data.
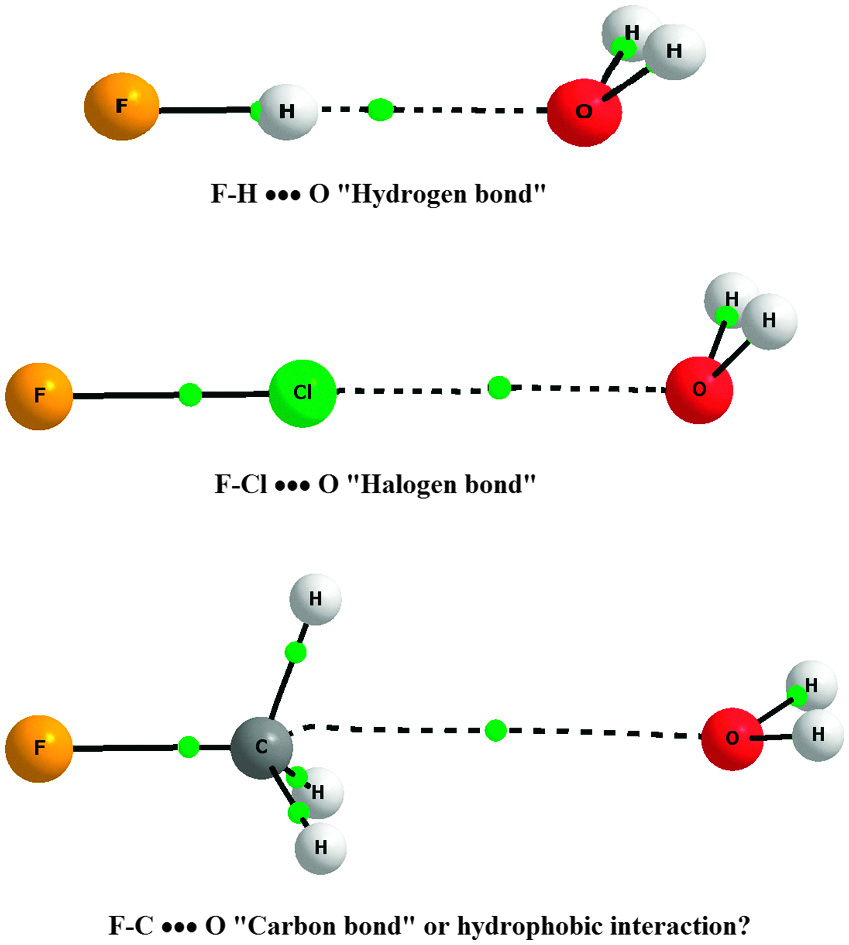
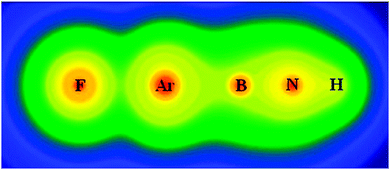
So what are these fantastic new molecules? They take the general form F–RG–BNR, where RG can be Argon, Krypton or Xenon, and R can be quite a variety of small groups. Interestingly, they found that the stability of the molecule was largely unaffected by the identity of the R group, indicating that it may be possible to study even more of them than were covered in this investigation.
All we need now is for somebody to work out how to synthesise these molecules in a spectroscopically useful environment, and perform the experiments to confirm the theoretical predictions. Experimental spectroscopists, consider the challenge issued!
By Victoria Wilton
Read the full details of this PCCP article:
Theoretical prediction of new noble-gas molecules FNgBNR (Ng = Ar, Kr, and Xe; R = H, CH3, CCH, CHCH2, F, and OH)
Jien-Lian Chen, Chang-Yu Yang, Hsiao-Jing Lin and Wei-Ping Hu
Phys. Chem. Chem. Phys., 2013, 15, 9701-9709
DOI: 10.1039/C3CP50447F
Comments Off on New, Stable Rare Gas Molecules Predicted!


![Remarks on time-dependent [current]-density functional theory for open quantum systems Remarks on time-dependent [current]-density functional theory for open quantum systems](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C3CP51127H)









 The latest citation data released by
The latest citation data released by