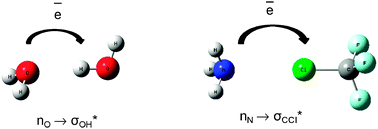
Hydrogen is the only element in the periodic table that is not truly part of a group, although it is often nominally assigned to group 1. All chemists are familiar with the concept of the hydrogen bond, but how many think of the halogen bond in the same light? How many are even aware of the halogen bond as a special entity, less still that the two interactions are to all intents and purposes the same thing?
In his recent paper, Grabowski uses theoretical techniques to show that both interactions are ruled by the same electrostatic mechanism. He also provides an excellent summary and comparison of the information currently known about the two interactions that indicates a clear progression in some bonding properties from hydrogen through to the heavy halogens.
He describes how the atomic volume of the halogen decreases as the positive charge on it increases, and that this effect is magnified by shortening the internuclear distance. This information accompanies the observation that the strength of the Lewis acid-base interaction increases with the increasing atomic number of the halogen involved, although with some exceptions, hydrogen bonds are generally stronger still.
This discovery clearly has important implications for our understanding of non-covalent molecular interactions, and our understanding of how best to classify hydrogen based on its bonding properties.
by Victoria Wilton
Read this HOT PCCP article today:
Hydrogen and halogen bonds are ruled by the same mechanisms
Sławomir J. Grabowski
DOI: 10.1039/C3CP50537E










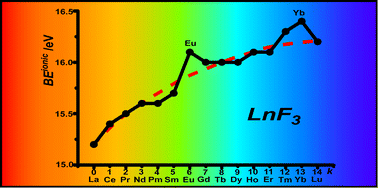 The complex electronic structure of the lanthanides and actinides is largely a mystery. The f-shell is not readily explained in our theoretical treatment of atoms and molecules, and coupled with the difficulty of obtaining readily interpreted experimental data. The result is that we are still struggling to understand the elements at the bottom of the periodic table. Xu and co-workers conduct a good and meticulous computational study of lanthanide trifluorides, and do significantly increase our understanding of the rare earth elements, albeit in simple molecules in the gas phase. My problem is that I work in aqueous solution, where lanthanide ions have not just three ligands—as the molecules investigated in this paper have—they have eight, nine or ten.
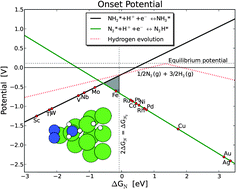
The complex electronic structure of the lanthanides and actinides is largely a mystery. The f-shell is not readily explained in our theoretical treatment of atoms and molecules, and coupled with the difficulty of obtaining readily interpreted experimental data. The result is that we are still struggling to understand the elements at the bottom of the periodic table. Xu and co-workers conduct a good and meticulous computational study of lanthanide trifluorides, and do significantly increase our understanding of the rare earth elements, albeit in simple molecules in the gas phase. My problem is that I work in aqueous solution, where lanthanide ions have not just three ligands—as the molecules investigated in this paper have—they have eight, nine or ten.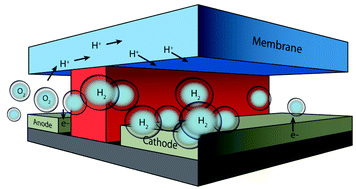 Segalman, Ager and co-authors present a versatile microfluidic test-bed for testing of integrated catalysis and mass transport components for energy conversion via water electrolysis in their recent PCCP Communication.
Segalman, Ager and co-authors present a versatile microfluidic test-bed for testing of integrated catalysis and mass transport components for energy conversion via water electrolysis in their recent PCCP Communication. The
The 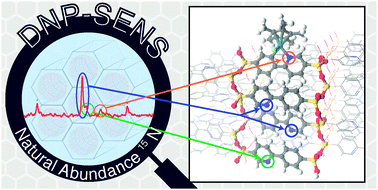 The development of new experimental methods to probe surface functionality is crucial to the understanding of functional materials. For the typically low concentrations of surface functional groups, traditional nuclear magnetic resonance (NMR) spectroscopy lacks the sensitivity to provide chemical information quickly.
The development of new experimental methods to probe surface functionality is crucial to the understanding of functional materials. For the typically low concentrations of surface functional groups, traditional nuclear magnetic resonance (NMR) spectroscopy lacks the sensitivity to provide chemical information quickly. 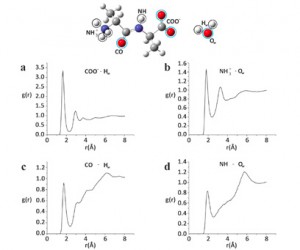
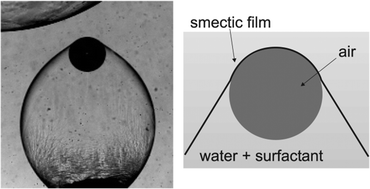 Sometimes you just have to sit back, read, and enjoy the ride. This is exactly the case with the work of Kirsten Harth and
Sometimes you just have to sit back, read, and enjoy the ride. This is exactly the case with the work of Kirsten Harth and 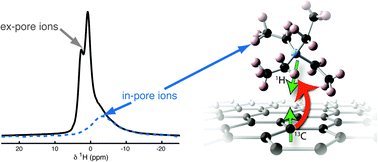 Developing alternative energy storage devices, such as supercapacitors, is of rising importance when facing today’s challenges of climate change and fossil fuel depletion. Supercapacitors typically employ porous carbon electrodes as they have large surface areas for ion electrosorption, good electronic conductivities and relatively low production cost. The design and improvement of supercapacitors is only possible with a detailed understanding of ion adsorption within porous carbon.
Developing alternative energy storage devices, such as supercapacitors, is of rising importance when facing today’s challenges of climate change and fossil fuel depletion. Supercapacitors typically employ porous carbon electrodes as they have large surface areas for ion electrosorption, good electronic conductivities and relatively low production cost. The design and improvement of supercapacitors is only possible with a detailed understanding of ion adsorption within porous carbon.