You can take a look at the excellent articles we have selected this week by clicking on the links below:
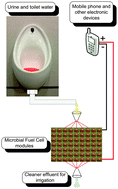 Waste to real energy: the first MFC powered mobile phone
Waste to real energy: the first MFC powered mobile phone
Ioannis A. Ieropoulos, Pablo Ledezma, Andrew Stinchcombe, George Papaharalabos, Chris Melhuish and John Greenman
DOI: 10.1039/C3CP52889H
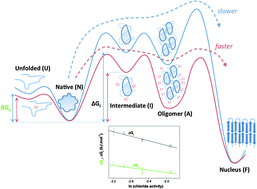
Modulation of the stability of amyloidogenic precursors by anion binding strongly influences the rate of amyloid nucleation
David Ruzafa, Francisco Conejero-Lara and Bertrand Morel
DOI: 10.1039/C3CP52313F
Electron transfer with azurin at Au–SAM junctions in contact with a protic ionic melt: impact of glassy dynamics
Dimitri E. Khoshtariya, Tina D. Dolidze, Tatyana Tretyakova, David H. Waldeck and Rudi van Eldik
DOI: 10.1039/C3CP51896E
Stabilizing effect of electrostatic vs. aromatic interactions in diproline nucleated peptide β-hairpins
Kamlesh Madhusudan Makwana, Srinivasarao Raghothama and Radhakrishnan Mahalakshmi
 DOI: 10.1039/C3CP52770K
DOI: 10.1039/C3CP52770K
Accurate adsorption energies of small molecules on oxide surfaces: CO–MgO(001)
A. Daniel Boese and Joachim Sauer
DOI: 10.1039/C3CP52321G
Solution-processed small molecule:fullerene bulk-heterojunction solar cells: impedance spectroscopy deduced bulk and interfacial limits to fill-factors
Antonio Guerrero, Stephen Loser, Germà Garcia-Belmonte, Carson J. Bruns, Jeremy Smith, Hiroyuki Miyauchi, Samuel I. Stupp, Juan Bisquert and Tobin J. Marks
DOI: 10.1039/C3CP52363B












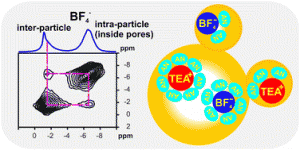
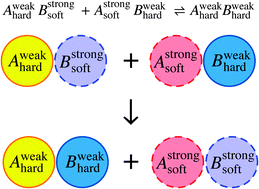 When I was perusing the ‘recently published’ pages for something to write about, I was moderately surprised to stumble on this article. It immediately caught my eye as being unlike anything I’d ever seen in
When I was perusing the ‘recently published’ pages for something to write about, I was moderately surprised to stumble on this article. It immediately caught my eye as being unlike anything I’d ever seen in 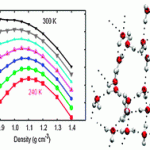
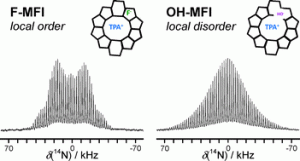
 Scientists at the
Scientists at the