 More than 50 % of our bodies are water. Plain and simple, we are more water than anything else. Following that statement it must be said that none of the water in our body is simply water. Excluding the content of your bladder, none of the water in your body is water as you have come to know it from your glass, rain, your local river and the oceans. Your body water surrounds cell membranes, microtubule, proteins, sugars, bones; all the smaller and larger biomolecules that make up our bodies. Not to mention the specific concentrations of salts and inorganic molecules that are required to run our bodies. We are beginning to understand the structure of water itself, and when water solvates simple ions. The work of Takis and co-workers increases the stakes and opens our mind to the specific solvations of small protein fragments.
More than 50 % of our bodies are water. Plain and simple, we are more water than anything else. Following that statement it must be said that none of the water in our body is simply water. Excluding the content of your bladder, none of the water in your body is water as you have come to know it from your glass, rain, your local river and the oceans. Your body water surrounds cell membranes, microtubule, proteins, sugars, bones; all the smaller and larger biomolecules that make up our bodies. Not to mention the specific concentrations of salts and inorganic molecules that are required to run our bodies. We are beginning to understand the structure of water itself, and when water solvates simple ions. The work of Takis and co-workers increases the stakes and opens our mind to the specific solvations of small protein fragments.
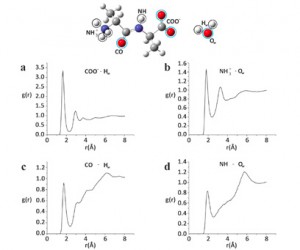
The research from the groups of Troganis and Melissas at the University of Ioannina is focused on simple dipeptides, and exploits molecular dynamic simulations, density functional theory based calculations and advanced nuclear magnetic resonance spectroscopy. This combination allows them to probe the solvation of the small peptide experimentally, and rationalize the findings by the theoretical approach. Specifically the distances between selected carbon atoms in the peptide structure and water molecules can be measured. The challenge in this approach is the continuous and rapid exchange of the water molecules.
To me, the most readily accessible data is the plots showing the probability of finding water oxygen and hydrogen atoms at a specific distance to selected groups in the peptide structure. Here, extracted from MD simulations. When experimental data can yield similar results, we will be able to start directly investigating how more than half of our bodies are made up.
The excellent work on the solvation of dipeptides is published in the PCCP paper titled:
Probing micro-solvation in “numbers”: the case of neutral dipeptides in water
Panteleimon G. Takis, Konstantinos D. Papavasileiou, Loukas D. Peristeras, Vasilios S. Melissas and Anastassios N. Troganis
Phys. Chem. Chem. Phys., 2013, 15, 7354-7362
DOI: 10.1039/C3CP44606A
by Thomas Just Sørensen











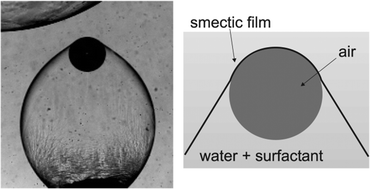 Sometimes you just have to sit back, read, and enjoy the ride. This is exactly the case with the work of Kirsten Harth and
Sometimes you just have to sit back, read, and enjoy the ride. This is exactly the case with the work of Kirsten Harth and