Perspectives
Redox and electrochemical water splitting catalytic properties of hydrated metal oxide modified electrodes
Richard L. Doyle, Ian J. Godwin, Michael P. Brandon and Michael E. G. Lyons
DOI: 10.1039/C3CP51213D, Perspective
Influence of adsorption thermodynamics on guest diffusivities in nanoporous crystalline materials
Rajamani Krishna and Jasper M. van Baten
DOI: 10.1039/C3CP50449B, Perspective
Electric-double-layer field-effect transistors with ionic liquids
Takuya Fujimoto and Kunio Awaga
DOI: 10.1039/C3CP50755F, Perspective
The role of interfacial charge transfer-type interactions in the decay of plasmon excitations in metal nanoparticles
Kenneth O. Aruda, Mario Tagliazucchi, Christina M. Sweeney, Daniel C. Hannah and Emily A. Weiss
DOI: 10.1039/C3CP51005K, Perspective
Original Research
Unusual molecular material formed through irreversible transformation and revealed by 4D electron microscopy
Renske M. van der Veen, Antoine Tissot, Andreas Hauser and Ahmed H. Zewail
DOI: 10.1039/C3CP51011E, Paper
A band Lanczos approach for calculation of vibrational coupled cluster response functions: simultaneous calculation of IR and Raman anharmonic spectra for the complex of pyridine and a silver cation
Ian H. Godtliebsen and Ove Christiansen
DOI: 10.1039/C3CP50283J, Paper
Spectral assignments and NMR parameter–structure relationships in borates using high-resolution 11B NMR and density functional theory
Oliver L. G. Alderman, Dinu Iuga, Andrew P. Howes, Kevin J. Pike, Diane Holland and Ray Dupree
DOI: 10.1039/C3CP50772F, Paper
Spectromicroscopy of pulses transporting alkali metal in a surface reaction
S. Günther, Hong Liu, T. O. Menteş, A. Locatelli and R. Imbihl
DOI: 10.1039/C3CP44478C, Paper
Dissecting the structural determinants for the difference in mechanical stability of silk and amyloid beta-sheet stacks
Senbo Xiao, Shijun Xiao and Frauke Gräter
DOI: 10.1039/C3CP00067B, Paper
First principles intensity calculations of the methane rovibrational spectra in the infrared up to 9300 cm−1
Michaël Rey, Andrei V. Nikitin and Vladimir G. Tyuterev
DOI: 10.1039/C3CP50275A, Paper
Quantitative studies of adsorbate dynamics at noble metal electrodes by in situ Video-STM
Yaw-Chia Yang and Olaf M. Magnussen
DOI: 10.1039/C3CP51027A, Paper
Ultra-slow dynamics in low density amorphous ice revealed by deuteron NMR: indication of a glass transition
Florian Löw, Katrin Amann-Winkel, Thomas Loerting, Franz Fujara and Burkhard Geil
DOI: 10.1039/C3CP50818H, Paper
Structures of hydrogen bond networks formed by a few tens of methanol molecules in the gas phase: size-selective infrared spectroscopy of neutral and protonated methanol clusters
Tomohiro Kobayashi, Ryunosuke Shishido, Kenta Mizuse, Asuka Fujii and Jer-Lai Kuo
DOI: 10.1039/C3CP50985K, Paper
Dependence on the structure and surface polarity of ZnS photocatalytic activities of water splitting: first-principles calculations
Xiangying Meng, Hai Xiao, Xiaohong Wen, William A. Goddard III, Song Li and Gaowu Qin
DOI: 10.1039/C3CP50330E, Paper
Changing the shape of molecular ions: photoisomerization action spectroscopy in the gas phase
B. D. Adamson, N. J. A. Coughlan, R. E. Continetti and E. J. Bieske
DOI: 10.1039/C3CP51393A, Paper
Structural polymorphism in self-assembled networks of a triphenylene based macrocycle
Kunal S. Mali, Matthias Georg Schwab, Xinliang Feng, Klaus Müllen and Steven De Feyter
DOI: 10.1039/C3CP51074C, Paper











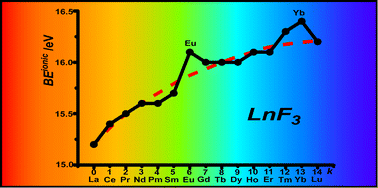 The complex electronic structure of the lanthanides and actinides is largely a mystery. The f-shell is not readily explained in our theoretical treatment of atoms and molecules, and coupled with the difficulty of obtaining readily interpreted experimental data. The result is that we are still struggling to understand the elements at the bottom of the periodic table. Xu and co-workers conduct a good and meticulous computational study of lanthanide trifluorides, and do significantly increase our understanding of the rare earth elements, albeit in simple molecules in the gas phase. My problem is that I work in aqueous solution, where lanthanide ions have not just three ligands—as the molecules investigated in this paper have—they have eight, nine or ten.
The complex electronic structure of the lanthanides and actinides is largely a mystery. The f-shell is not readily explained in our theoretical treatment of atoms and molecules, and coupled with the difficulty of obtaining readily interpreted experimental data. The result is that we are still struggling to understand the elements at the bottom of the periodic table. Xu and co-workers conduct a good and meticulous computational study of lanthanide trifluorides, and do significantly increase our understanding of the rare earth elements, albeit in simple molecules in the gas phase. My problem is that I work in aqueous solution, where lanthanide ions have not just three ligands—as the molecules investigated in this paper have—they have eight, nine or ten.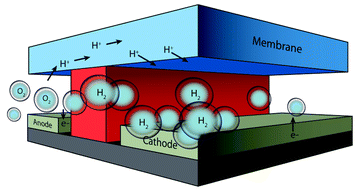 Segalman, Ager and co-authors present a versatile microfluidic test-bed for testing of integrated catalysis and mass transport components for energy conversion via water electrolysis in their recent PCCP Communication.
Segalman, Ager and co-authors present a versatile microfluidic test-bed for testing of integrated catalysis and mass transport components for energy conversion via water electrolysis in their recent PCCP Communication. The
The 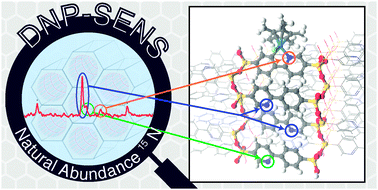 The development of new experimental methods to probe surface functionality is crucial to the understanding of functional materials. For the typically low concentrations of surface functional groups, traditional nuclear magnetic resonance (NMR) spectroscopy lacks the sensitivity to provide chemical information quickly.
The development of new experimental methods to probe surface functionality is crucial to the understanding of functional materials. For the typically low concentrations of surface functional groups, traditional nuclear magnetic resonance (NMR) spectroscopy lacks the sensitivity to provide chemical information quickly.