The world of organic dyes is extremely dynamic, and vast amounts of research are performed on all aspects of organic colorants. That said, the number of chromophores is very static, and has been for decades. Rarely is a completely new dye seen, most developments are perturbations of known dye structures. A very interesting perturbation of a xanthenium dye is reported in a recent paper by Kamino et al., where they have fused two cationic xanthenium systems into a single dye.
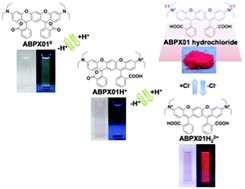
A consequence of fusing two rhodamines into a single molecule is the doubling of all charges. The new dye is a dication in its most potent form, a species that evidently is highly solvent sensitive. More interesting, two peripheral phenyl groups are present in the system. The short distance between these results in hindered rotation. Two different isomers, defined by the phenyl substituents, can be isolated and they have significantly different properties.
The new dyes, which have been synthesised and characterised by Kamino et al. are exciting, as they allow for the investigation of how an enlarged pi-system changes the optical properties. As well as how the phenyl group, present in all fluoresceins and rhodamines, can affect their performance as stains and labels for the biological and medical sciences. Thus providing a new handle in the search for new and optimised fluorescent dyes.
by Dr Thomas Just Sørensen
Check out this fascinating PCCP article now:
A red-emissive aminobenzopyrano-xanthene dye: elucidation of fluorescence emission mechanisms in solution and in the aggregate state
Shinichiro Kamino, Atsuya Muranaka, Miho Murakami, Asana Tatsumi, Noriyuki Nagaoka, Yoshinao Shirasaki, Keiko Watanabe, Kengo Yoshida, Jun Horigome, Seiji Komeda, Masanobu Uchiyama and Shuichi Enomoto
Phys. Chem. Chem. Phys., 2013, 15, 2131-2140
DOI: 10.1039/C2CP43503A










