An extended periodic table with 54 predicted elements has been mapped out and is reported in Physical Chemistry Chemical Physics this week.
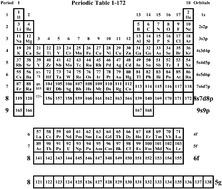
The extra 54 super heavy elements predicted by Pyykkö may exist under extreme conditions with very short lifetimes due to radioactive decay but have not yet been synthesised. The work shows how the rules of quantum mechanics and relativity function in determining chemical properties, says Pyykkö, such as the potentially record-high oxidation states that he predicts.
Mendeleev’s classification of elements into groups and periods was given a solid theoretical foundation by the development of chemical quantum mechanics in the early 20th century. Quantum mechanical rules describing interactions of electrons and protons dictate electronic structures for elements which give rise to the properties of elements and therefore their positions in the periodic table.
Read the paper now:
A suggested periodic table up to Z ≤ 172, based on Dirac–Fock calculations on atoms and ions
P Pykko, Phys. Chem. Chem. Phys, 2010
DOI: 10.1039/ c0cp01575j










