R.S. Mulla1
1) Sygnature Discovery, BioCity, Pennyfoot Street, Nottingham, NG1 1GR
r.mulla@sygnaturediscovery.com
ORCID:0000-0001-9161-4486
1.1 Purpose
As with all chemistry, the success of reactions run in flow requires a proper understanding and control of the operating
temperature. Often, the reported temperature is that of the heat transfer medium (heating, cooling bath or block), which
will differ from the temperature of the contents of a flow line, especially when highly exothermic or endothermic reactions
are conducted. Other factors which affect the temperature difference include but are not limited to: the residence times
of any preheating/precooling loops, their material of construction, surface to volume ratio and wall thickness. Therefore,
in-line temperatures should be measured and controlled directly to negate these factors.
In this post, the modification of readily available small-scale flow chemistry equipment (T-pieces, fittings) to accept
a standard, commercially available thermocouple for in-line temperature measurements is described. The resulting
apparatus is intended to accept incoming reagents into a T-piece port directly opposite the port in which the thermocouple
sits, since there is evidence that such a configuration results in more accurate temperature measurements [1].
1.2 What problem does this work address?
One approach to in-line thermometry is the use of a thermocouple or a resistive thermocouple device (RTD) probe
fixed into place by means of a Swagelok-type fitting [1, 2]. While this is appropriate for many applications, alternative
approaches to construction are desirable for a number of reasons. For instance, much of the chemistry our team runs in
flow employs is temperature and residence time critical, on a small scale and is highly dependent on mixing quality. In our
case, mixing quality is usually controlled via use of narrow-bore T-pieces with internal diameters of 0.5 mm coupled to
tubing of internal diameter 1 mm. Incorporating an inline thermocouple constructed using the smallest readily available
Swagelok T-pieces (=1.6 mm, Vint100 μL, relatively large for our purposes) can increase residence times significantly
and affect mixing compared to a device with a smaller internal diameter and volume.
In addition, the stainless steel construction of inline thermocouples incorporating Swagelok-type T-pieces limits the
scope of chemistry they can be used to monitor. As an example, they are incompatible with chloride rich solutions
or hydrochloric acid. Therefore, the use of more chemically resilient materials for constructing the inline section e.g.
polymers such as PTFE, ETFE, PFA or PPS is advantageous. These materials are also thermally insulating, so heat
transfer into an analyte from the surroundings (e.g. when a reactor is immersed in a coolant) as it passes through an inline
thermocouple made using an appropriate polymer will be reduced compared to an in-line thermocouple made using
stainless steel. To the author’s knowledge, no in-line thermocouple devices made from chemically resistant polymeric
materials, with the requisite narrow diameter and the ability to accept a thermocouple are commercially available; a
bespoke solution is necessary. While the use of inline thermocouples is extensively documented in the literature, a
detailed method for their assembly is not, making the publication of such a desirable.
By adapting these instructions to different components, the below may also serve as a blueprint for additional miniaturisation
beyond that described. For instance, T-pieces of 0.25 mm internal diameter may be used to construct in-line
thermocouples for microfluidic applications, and the customisation of flangeless fittings in the manner described enables
use of a far wider range of small-form thermocouple devices beyond those compatible with commercially available fittings.
1.3 Materials
- A Vernier gauge for drill bit selection (particularly if components other than those described are to be used)
- Appropriately sized drill bits (2.1 mm and 0.45 mm were suitable for the modifications described)
- A pillar drill, pin vice or similar
- 1x 90°T-piece (BOLA F707-14, with an internal diameter of 0.5 mm was used in this work)
- UNF 1/4-28 flangeless fittings for 1/16” tubing (IDEX P245/P200N used. For increased operating pressures and
vibration resistance, IDEX lines P-246X and P-259X may be more appropriate).
– If additional miniaturisation is desired, the use of UNF 10-32 fittings in conjunction with a suitable T-piece may
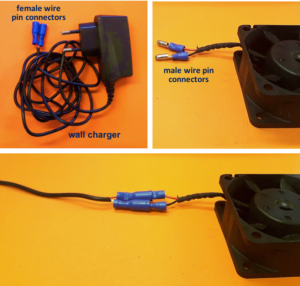
be considered. - An appropriately insulated thermocouple wire or RTD. Note that:
– Insulation should be made from material resistant to any reagent or solvent used in your application. PTFE or
PFA are recommended.
– The shape of the insulation around the wire must be cylindrical to ensure the final assembly seals properly on
fabrication.
– For this work, Sterling Sensors TWSSTK00002M00P1 was used for the above reasons. In addition, its exposed
end was advantageous to us, since it drastically shortened the response time of the probe, making it
suited to reading rapid (across <10 s ) temperature changes. If the exposed end presents a compatibility issue,
a hermetically sealed thermocouple (e.g. Sterling Sensors TWHSEK00002M0AP7) may be used instead.
– The choice of temperature sensor will depend on your application. For fast response times at extreme of
temperatures at low cost, a K type thermocouple is suggested. For more accurate measurements across a
long time period at the expense of response time, a resistive thermocouple device (RTD) such as a Pt100
probe may be more appropriate [3, 4].
1.4 Procedure
1. Measure the thermocouple insulation diameter and the diameter of the orifices of the T-piece if not known. Select a
drill bit of equivalent size to the insulation diameter of the thermocouple wire (bit A) and one that is slightly smaller
(0.05-0.3 mm) than the diameter of the T-piece orifices (bit B).
2. Drill through the holes of the flangeless fitting nut and ferrule to enlarge them using bit A in an appropriate drill.
Ensure that drilling takes place parallel to the threads of the nut and perfectly through the centre of the ferrule.

3. Thread the thermocouple wire through the nut and ferrule making sure the end protrudes about 1-2 mm beyond
the face of the ferrule. Additional enlargement of the nut bore to a diameter slightly larger than that of bit A may be
necessary for smoother threading of the thermocouple insulation through the nut body.

4. Thread bit B through the centre orifice of the T-piece, to create a depth stop.

5. Drill to enlarge the centre orifice of the T-piece using bit A while bit B is in place. Proper drilling depth will be
indicated by an increase in resistance from the drill upon contact with the drill already inserted into the T-piece.
Keep bit B in place for the next step and clear away any residue from drilling.

6. With the thermocouple ferrule separated from the nut, push the thermocouple through the enlarged orifice on the
modified T-piece until it hits the depth stop formed by bit B. Do not apply excessive pressure.

7. Form a sealed fitting between the thermocouple and T-piece by rotating the T-piece around the nut thread to finger
tightness.
8. Withdraw bit B from the T-piece.

9. Attach the thermocouple to a suitable reader and check for leaks in your fluidic system, connecting the input
stream directly opposite to the thermocouple connection and the outlet at 90°to it. If leaks are detected, they may
be plugged by applying epoxy resin to the affected area (ensure that the resin is chemically compatible with the
reagents and solvents to be used in experimental work). If leakage through the thermocouple insulation is observed
as evidenced by the deposition of material at or near the termination, it is likely that the sealing has failed and that
the thermocouple wire should be replaced.
1.5 Notes
We have found in-line thermocouples made in this manner will function across multiple, extended runs, provided that
connection between the T-piece and the thermocouple is not repeatedly loosened and tightened. Pressure testing also
indicates that the design as described tolerates pressures of at least 8 bar. Even though it is more accurate, the above
thermocouple construction can result in increased back pressures for fluid streams because of the path the liquid stream
must take around a bend. If this is problematic, an in-line thermocouple in a “perpendicular” configuration may be
constructed in a similar manner to the above. This is demonstrated in the panel below which also demonstrates the use
of IDEX P-246X and P-259X fittings in the construction of the thermocouple.
References
[1] V. Nuredin, L. Schulz, N. Kockmann and T. Röder, J Flow Chem, 2025.
[2] A. Hafner, P. Filipponi, L. Piccioni, M. Meisenbach, B. Schenkel, F. Venturoni and J. Sedelmeier, Org. Process Res.
Dev., 2016, 20, 1833–1837.
[3] RTD vs Thermocouple (Pt100 vs Thermocouple), https://www.sterlingsensors.co.uk/rtd_vs_thermocouple.
[4] Thermocouple vs RTDs, https://www.dwyeromega.com/en-us/resources/rtd-vs-thermocouple.
1.6 Acknowledgments
RSM thanks James Cochrane, Aaron Rigby and Prof. Glenn Walker for feedback on this work.