1Sabrina Banella, 1Gaia Colombo and 2Claudio Nastruzzi
Email: bnlsrn@unife.it ; clmgai@unife.it ; nas@unife.it
1 Department of Life Sciences and Biotechnology and 2 Department of Chemical, Pharmaceutical and Agricultural Sciences, University of Ferrara, Ferrara, Italy.
Why is this useful?
Considering chemical bulk reactions that take place in aqueous phase, it is always necessary to control the temperature in a range usually comprised between few degrees Celsius (typically obtained by ice bath) up to a temperature near to the boiling point of water (i.e., 70-90 °C). This aspect is also true for reactions carried out in microfluidic conditions and in this respect, it could be sometimes tricky to control the temperature of liquids pumped to the chip by a syringe pumps.
Syringe-pumps have indeed the disadvantage to be pumping systems in which the temperature of the liquid is difficult to control. This aspect is particularly relevant for some microfluidic applications in which one or more liquid phases are pumped through the chip.
For instance, in recent years, microfluidic platforms have been largely employed for the production of liposomes and other supramolecular colloidal systems. Nastruzzi et al. have indeed reviewed the use of microfluidic chips for the production of liposomes [1].
Despite the large applicability and usefulness of syringe pumps, they could present some drawbacks with respect to fine control of the temperature. In many applications, maintaining a specific temperature is indeed crucial, as in the case of saturated (i.e., hydrogenated) phospholipids that are usually soluble only at temperature above 40-50 °C. Moreover, it has to be underlined that the whole preparation process of liposomes by hydrogenated phospholipids is to be carried out at temperature above the phase transition temperature of the lipid (Tm) [2]. The control of temperature is also required for other reactions carried out in microfluidics when the reagent requires to be manipulated at 4-5 °C or in a particular range of temperature [3].
In order to possibly solve the problem of temperature control of liquids delivered by a syringe pump, we propose here a new, very low-cost and alternative approach, with respect to another system presented on Chips and Tips [4] or in the current literature [5]. The device here presented is indeed easily applicable to any type of syringe and syringe pumps.
The idea is to use fluorinated ethylene propylene (FEP) tubes that, owing to their shape-memory behavior, can be heated and molded in the form of a spiral. We named these hand-made systems as “Graham temperature controller” (GTC).
What do I need?
- Polypropylene syringe (typical volume between 5 and 50 ml) (BD Switzerland Sarl, Vaud, Switzerland)
- 21-gauge hypodermic needle (0.80 x 40 mm; 21G x 1 ½”; PIC)
- Tube #1 – PTFE tubing, ID 0.8 mm, OD 1.58 mm (Z609714, Sigma-Aldrich, Missouri, USA)
- Tube #2 – Tube FEB Nat 1/8 x 0.062 x 20ft (WO#0556708, Idex Health & Science, Washington, USA)
- Tube #3 – Tube FEP Nat 3/16 x 0.125 x 20ft (Upchurch Scientific, Washington, USA)
- As template for spiraling the tubes different pieces of glassware and plastic commonly present in the lab can be used. For example: 10 ml test tubes with NS neck (DWK Life Sciences, New Jersey, USA) with an external diameter of 1.8 mm; 50 ml centrifuge disposable plastic conical tube plastic with an external diameter of 3 cm – plastic powder container with external diameter of 3.8 cm
- Heat gun (Black & Decker BD1666 2 heat swing handle paintstripper or any other similar tool)
- Glass becker fitting the external diameter of the Graham like temperature controller
- Heating plate or ice water bath
- 100 ml becker Scott
What do I do?
- After warming up the FEP tubes by a heat gun till the polymer softens (be careful not to burn your fingertips; in case use leather gloves), the tube is molded and spiraled around a template (see list of templates in the What do I need? section). Thereafter, the spiraled tube is rapidly cooled down to quench the given shape, putting it into an ice-cold water bath. In this way, the rapid cooling of the tubing allows to retain the shape given since it was warm. As said, this feature is due to the peculiar mechanical properties of FEP polymer that is characterized by a shape-memory behavior.
- Once the GTC is produced, it can be connected to the syringe pump (via the Luer lock male connector on one side) and to the chip (via the inlet tube of the microfluidic network). Typically, the connecting tubes are produced by smaller diameter tubes such as Tube #1 (see Figure).
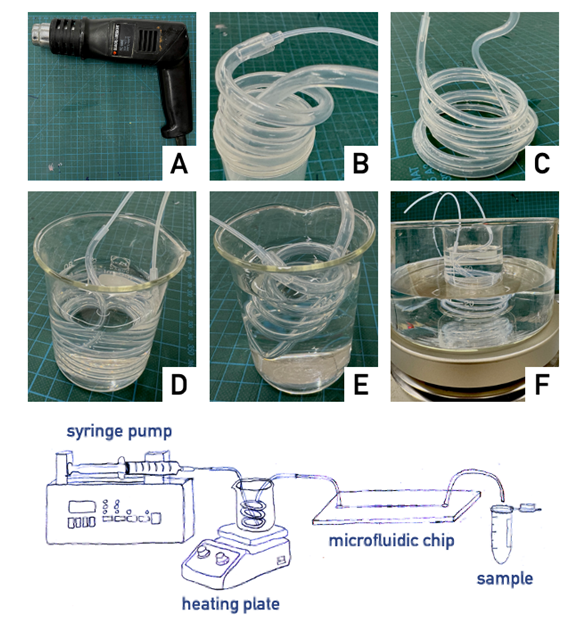
- The GTC can be fitted in any suitable liquid container, such as a 100 ml glass becker or a 50 ml plastic centrifuge tube. As an example, if conditions above the room temperature are required (40-80 °C), the becker is then placed on a heating plate or in a boiling water bath. Analogously, if conditions lower than room temperature are required, an ice-cold water bath can be employed.
- Before operating the syringe pump, typically the liquid is left into the GTC for 10 minutes in order to assure that the liquid in the spiraled tube reaches the desired temperature.
This tip can be easily tuned varying the volume of the spiraled tube, using tube sections of different lengths or tubes with larger internal diameter.
| Tube type | Tube length (mm) | ||
| 10 | 20 | 30 | |
| #1 | 50 | 100 | 150 |
| #2 | 184 | 368 | 552 |
| #3 | 750 | 1500 | 2250 |
Reported data indicate the volume, expressed in microliters, contained by different length of tubings.
Application note
As an example of application of the GTC, below is reported the experimental procedure relative to the preparation of liposomes composed of hydrogenated phosphatidyl choline (HPC), using an ethanol injection protocol in a chip with a flow focusing geometry [1].
Experimental procedure
Typically, a “cross-flow” chip is employed for the preparation of liposomes. Liposomes were prepared by injecting a lipid mixture (HPC 90 mM, Phospholipon 90 H, Lipoid GMBH, Ludwigshafen, Germany) dissolved in a mixture of ethanol/water (95:5, v/v), heated at 60 °C by the GTC. The lipid solution was injected into the central channel of the microfluidic network of the chip, whereas water was injected into two oblique side channels intersecting with the central one. The flow rate ratio (FRR), defined as the ratio between the water volumetric flow rate and the ethanol volumetric flow rate, was varied from 10 to 50. Liposomes can be prepared by changing also the total flow rate (TFR), typically from 18.75 to 75.00 μl/min.
References
- Carugo D, Bottaro E, Owen J, Stride E, Nastruzzi C. Liposome production by microfluidics: potential and limiting factors. Sci Rep., 6:25876 (2016).
- Chen W, Duša F, Witos J, Ruokonen SK, Wiedmer SK. Determination of the Main Phase Transition Temperature of Phospholipids by Nanoplasmonic Sensing. Sci Rep., 8(1):14815 (2018).
- Shen J, Liao J, Liu H, Liu C, Li C, Cheng H, Yang H, Chen H. A low-temperature digital microfluidic system used for protein-protein interaction detection. Lab Chip., 23(20):4390-4399 (2023).
- Capretto L, Mazzitelli S, Nastruzzi C. An easy temperature control system for syringe pumps. Chips and Tips (2008).
- Cantoni F, Werr G, Barbe L, Porras AM, Tenje M. A microfluidic chip carrier including temperature control and perfusion system for long-term cell imaging. HardwareX, 10:e00245 (2021).










