Abdul Wasay, Dan Sameoto
Department of Mechanical Engineering, University of Alberta, Edmonton, T6G 2R3, CANADA.
Email: sameoto@ualberta.ca
Why is it useful?
Electrodes have been used for driving electrochemical reaction on various Lab on chip applications, running electroosmotic pumping or simple sensing of current or voltages. With the recent thrust to move to thermoplastics, simple electrodes have been patterned at low temperatures using vacuum plasma sputtering via intermediate masks1 or ink electrodes2. While, wire-bonding or soldering have been the standard protocols for providing interconnections to the bond pads on silicon or glass chips, the temperatures in these cases are often beyond the glass transition or even melting temperatures of most thermoplastic substrates, which leads to defects and ineffective connection. Other techniques such as conductive epoxies can be problematic due to longer cure times, permanent fixtures and potentially low uniformity of conductive properties which are affected by sintering times and temperatures.
We present a simple soldering technique using low temperature melting metals that is compatible with nearly any thermoplastic substrate and thin-film electrodes.
- Field’s metal (eutectic alloy of 32.5% Bi, 51% In, 16.5% Sn) preferred for low toxicity, although lead based alternatives exist.
- Syringe connected to tygon tube
- Copper wire
- Aluminum foil dish
- Electrodes patterned on thermoplastic substrate (here, polystyrene with ~ 20 nm thick Au electrodes)
Tools
- Hot Plate
- Shearing scissor
- Hot glue gun (in this case a Mastercraft dual temperature glue gun, operated at low temperature setting)
What do I do?
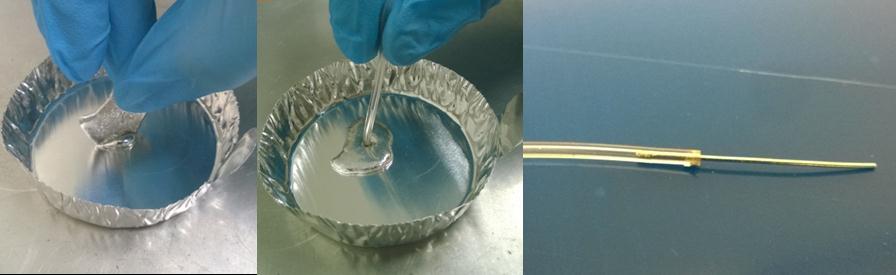
To extrude the Fields metal into a thin filament, (Fig.2)
- melt the Fields metal (ROTO1443; Tm=62.2oC, typical resistivity ~520nΩ-m) in an aluminum foil dish. The metal is very soft, so bolt cutters can easily remove smaller portions for melting if desired.
- use a suitable diameter and length tygon tube and pull the molten metal into it using a syringe – it will freeze within a few inches (1/32” inner diameter tube shown here).
- allow it to cool to room temperature (less than a minute) and then pull out the filament.
Soldering Process,(Fig.3.)
- Hold the Field’s metal filament with a hot glue gun used for lower melting point glues (measured tip temperature ̴120 C) over the electrode patterned petri dish and melt it over the electrodes.
- Solder the copper wire with the Field’s metal. After soldering, excess metal can be removed from the hot glue gun tip with paper towel.
The resultant solder is strong enough to be used for mainstream lab on chip applications. They can be removed by a strong tug, under which case the thin electrodes on plastics could be stripped.
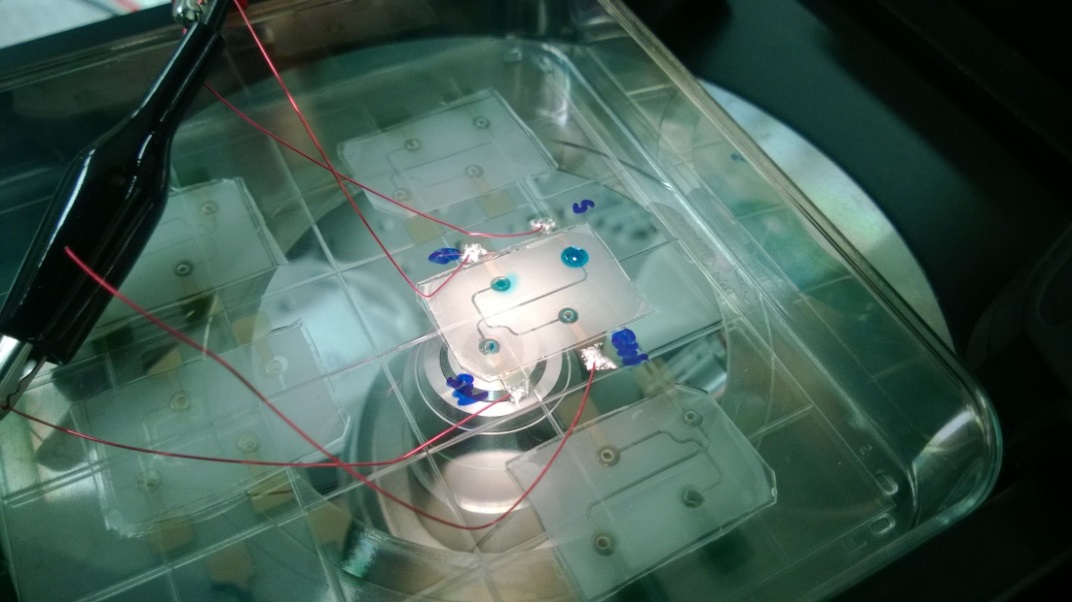
Fig.4. Gecko adhesives based reversibly bonded4 capillary electrophoresis device with integrated electrodes
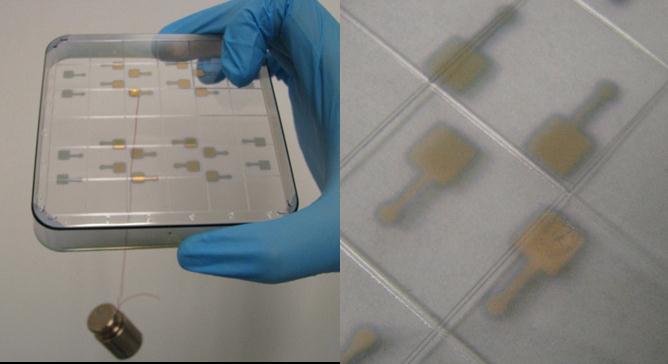
Fig.5. a) Demonstration of solder strength b) small electrode stripping as observed when the solder is removed by a strong tug
What else should I know?
While there are quite a few options of low melting temperature metals to choose from, most of them contain heavy metal like lead or cadmium, which may ideally be avoided. While Field’s metal is relatively expensive due to the indium content, the solders can be recovered for reuse in the lab and in general is a better option for electrically connecting thin-film electrodes to temperature sensitive substrates in a quick, reliable fashion.
Watch the video here: Low Temperature Melting Metal Solders For Electrical Interconnects On Plastics
References:
1 A. Toossi, M.Sc, University of Alberta, 2012.
2 C. E. Walker, Z. Xia, Z. S. Foster, B. J. Lutz and Z. H. Fan, ELECTROANALYSIS, 2008, 20, 663-670.
3 http://www.rotometals.com/product-p/lowmeltingpoint144.htm
4 A. Wasay and D. Sameoto, Lab Chip, 2015, (DOI:10.1039/C5LC00342C).