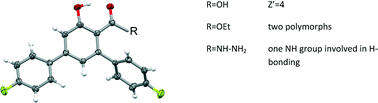Pseudosymmetry, polymorphism and weak interactions: 4,4′′-difluoro-5′-hydroxy-1,1′:3′,1′′-terphenyl-4′-carboxylic acid and its derivatives
Anita M. Owczarzak, Seranthimata Samshuddin, Badiadka Narayana, Hemmige S. Yathirajan and Maciej Kubicki
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41639A
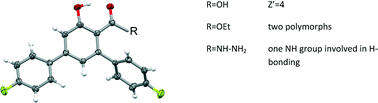
Free until 25 November 2013.
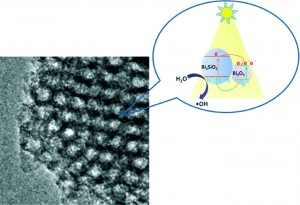
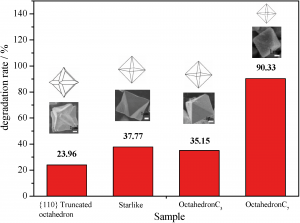
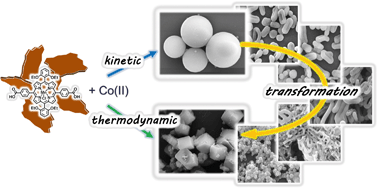
Morphological diversity of Mn(III) metalloporphyrin-based nano- and microsized CPAs assembled via kinetic and thermodynamic controls and their application in heterogeneous catalysis
Kyung Yeon Lee, Young Sun Lee, Sundol Kim, Hoang Mai Ha, Sang-Eun Bae, Seong Huh, Ho Gyeom Jang and Suk Joong Lee
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41072B
Free until 25 November 2013.
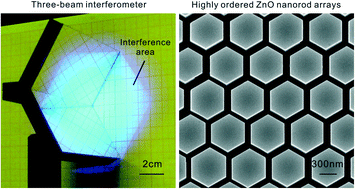
High-throughput fabrication of large-scale highly ordered ZnO nanorod arrays via three-beam interference lithography
Xiang Chen, Xiaoqin Yan, Zhiming Bai, Yanwei Shen, Zengze Wang, Xianzi Dong, Xuanming Duan and Yue Zhang
CrystEngComm, 2013,15, 8416-8421
DOI: 10.1039/C3CE41558A
Free until 25 November 2013.
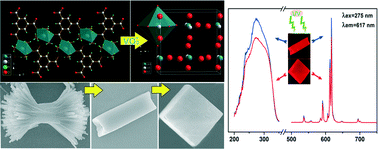
Novel synthesis and luminescence properties of t-LaVO4:Eu3+ micro cube
Baiqi Shao, Qi Zhao, Ning Guo, Yongchao Jia, Wenzhen Lv, Mengmeng Jiao, Wei Lü and Hongpeng You
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41456F
Free until 25 November 2013.
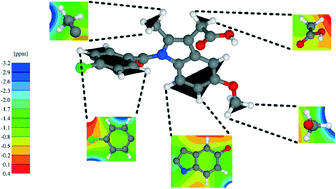
An NMR crystallography DFT-D approach to analyse the role of intermolecular hydrogen bonding and π–π interactions in driving cocrystallisation of indomethacin and nicotinamide
Dmytro V. Dudenko, Jonathan R. Yates, Kenneth D. M. Harris and Steven P. Brown
CrystEngComm, 2013,15, 8797-8807
DOI: 10.1039/C3CE41240G
Free until 25 November 2013.
Substitution at the metal center of coordination polymers in single-crystal-to-single-crystal (SC-SC) transformation
Subhadip Neogi, Susan Sen and Parimal K. Bharadwaj
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41257A
Free until 25 November 2013.
A new synthetic approach to force bond formation between a transition metal complex and a thiostannate anion: solvothermal synthesis and crystal structure of [Co2(cyclam)2Sn2S6]·2H2O
Christoph Zeisler, Christian Näther and Wolfgang Bensch
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE40976G

Free until 25 November 2013.
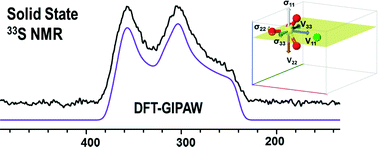
Structural assessment of anhydrous sulfates with high field 33S solid state NMR and first principles calculations
Peter J. Pallister, Igor L. Moudrakovski, Gary D. Enright and John A. Ripmeester
CrystEngComm, 2013,15, 8808-8822
DOI: 10.1039/C3CE41233D
Free until 25 November 2013.
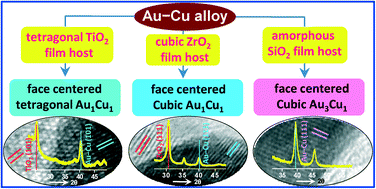
Crystal structure tailoring of Au–Cu alloy NPs using the embedding film matrix as template
Sourav Pramanik, Manish Kr Mishra and Goutam De
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41342J
Free until 25 November 2013.
Chelation-driven fluorescence deactivation in three alkali earth metal MOFs containing 2,2′-dihydroxybiphenyl-4,4′-dicarboxylate
Damien Rankine, Tony D. Keene, Christopher J. Sumby and Christian J. Doonan
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41253A
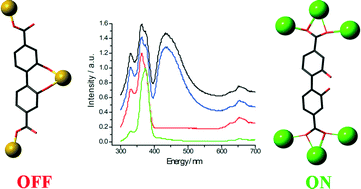
Free until 25 November 2013.
Polymer guided synthesis of Ni(OH)2 with hierarchical structure and their application as the precursor for sensing materials
Guoxing Zhu, Yuanjun Liu, Chunyan Xi, Chunlin Bao, Huan Xu, Xiaoping Shen and Xiaolu Zhu
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41614C
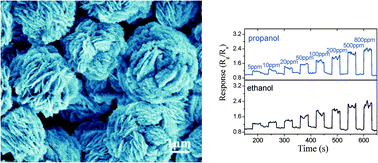
Free until 25 November 2013.
Electrodeposited CoSn2 on nickel open-cell foam: advancing towards high power lithium ion and sodium ion batteries
José R. González, Francisco Nacimiento, Ricardo Alcántara, Gregorio F. Ortiz and José L. Tirado
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41368C
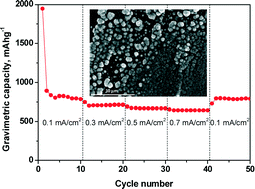
Free until 25 November 2013.
Activation of metal–organic framework materials
Joseph E. Mondloch, Olga Karagiaridi, Omar K. Farha and Joseph T. Hupp
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41232F
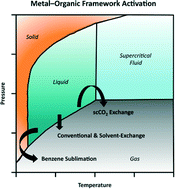
Free until 25 November 2013.
Infinite stacking of alternating polyfluoroaryl rings and bromide anions
Vickery L. Arcus, Daniel R. Bernstein, Cameron W. Crombie and Graham C. Saunders
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41582A
Free until 25 November 2013.
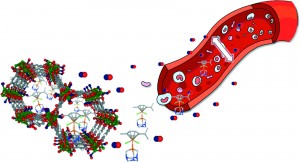
A biocompatible calcium bisphosphonate coordination polymer: towards a metal-linker synergistic therapeutic effect?
Elsa Alvarez, Alfonso Garcia Marquez, Thomas Devic, Nathalie Steunou, Christian Serre, Christian Bonhomme, Christel Gervais, Isabel Izquierdo-Barba, Maria Vallet-Regi, Danielle Laurencin, Francesco Mauri and Patricia Horcajada
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41346B

Free until 25 November 2013.
Crystal engineering the clathrate hydrate lattice with NH4F
Kyuchul Shin, Igor L. Moudrakovski, Mehdi D. Davari, Saman Alavi, Christopher I. Ratcliffe and John A. Ripmeester
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE41661E

Free until 25 November 2013.
Comments Off on HOT articles for October











![Pi-Bonded molecular wires: self-assembly of mixed-valence cation-radical stacks within the nanochannels formed by inert tetrakis[3,5-bis(trifluoromethyl)phenyl]borate anions Pi-Bonded molecular wires: self-assembly of mixed-valence cation-radical stacks within the nanochannels formed by inert tetrakis[3,5-bis(trifluoromethyl)phenyl]borate anions](https://blogs.rsc.org/ce/files/2013/11/c3ce41719k-ga-300x110.jpg)

 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently she is writing a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently she is writing a book on chemicals from plants.