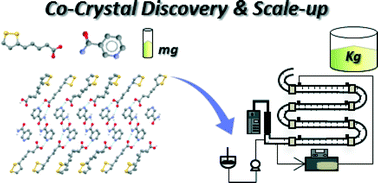
From discovery to scale-up: α-lipoic acid:nicotinamide co-crystals in a continuous oscillatory baffled crystalliser
Lihua Zhao, Vishal Raval, Naomi E. B. Briggs, Rajni M. Bhardwaj, Thomas McGlone, Iain D. H. Oswald and Alastair J. Florence
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE00154K
Free to access until 8th May 2014
Pharmaceutical co-crystals – are we there yet?
N. Blagden, S. J. Coles and D. J. Berry
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE00127C
Free to access until 8th May 2014
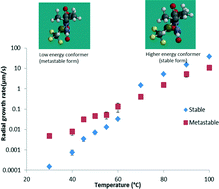
Nucleation and crystal growth of amorphous nilutamide – unusual low temperature behavior
Niraj S. Trasi and Lynne S. Taylor
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE00118D
Free to access until 8th May 2014
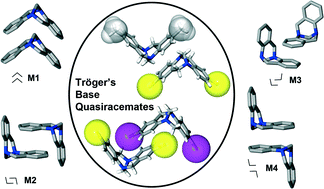
Tröger’s base quasiracemates and crystal packing tendencies
Jacob T. Cross, Nicholas A. Rossi, Mateusz Serafin and Kraig A. Wheeler
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE00323C
Free to access until 8th May 2014
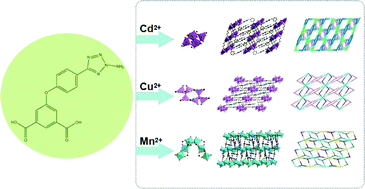
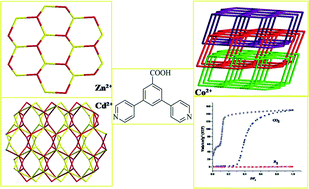
Three metal–organic frameworks based on the semirigid V-shaped 5-(3-amino-tetrazole-5-phenoxy)-isophthalic acid ligand: syntheses, topological structures and properties
Kang Liu, Yu Peng, Fen Yang, Dingxuan Ma, Guanghua Li, Zhan Shi and Shouhua Feng
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE00105B
Free to access until 16th April 2014
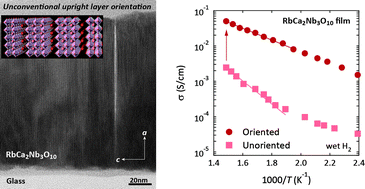
Unconventional upright layer orientation and considerable enhancement of proton–electron conductivity in Dion–Jacobson perovskite thin films
Tomohiko Nakajima, Kiyoshi Kobayashi, Kentaro Shinoda and Tetsuo Tsuchiya
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE42418A
Free to access until 16th April 2014
The role of a liquid in “dry” co-grinding: a case study of the effect of water on mechanochemical synthesis in a “L-serine–oxalic acid” system
Evgeniy A. Losev and Elena V. Boldyreva
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE42321B
Free to access until 11th April 2014
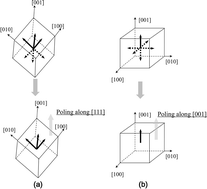
Achieving single domain relaxor-PT crystals by high temperature poling
Fei Li, Linghang Wang, Li Jin, Zhuo Xu and Shujun Zhang
CrystEngComm, 2014,16, 2892-2897
DOI: 10.1039/C3CE42330A
Free to access until 11th April 2014
Synthesis, characterization and selective hysteretic sorption property of metal–organic frameworks with 3,5-di(pyridine-4-yl)benzoate
Pei-Pei Cui, Yue Zhao, Gao-Chao Lv, Qing Liu, Xiao-Liang Zhao, Yi Lu and Wei-Yin Sun
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE42260G
Free to access until 4th April 2014
Investigation of the effect of liquid–liquid phase separation (LLPS) on nucleation and different growth stages of vanillin and bulk growth of defect-free single crystals from aqueous solution – a new approach
P. Parimaladevi, C. Kavitha and K. Srinivasan
CrystEngComm, 2014,16, 2565-2569
DOI: 10.1039/C3CE42416B
Free to access until 4th April 2014
Synthesis and luminescence of uniform europium-doped bismuth fluoride and bismuth oxyfluoride particles with different morphologies
Alberto Escudero, Elisa Moretti and Manuel Ocaña
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE42462F
Free to access until 4th April 2014
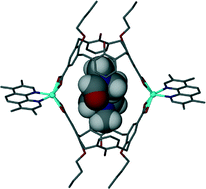
Metal–organic calixarene capsules: the evolution of controlled assembly
Piotr P. Cholewa and Scott J. Dalgarno
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE42169D
Free to access until 4th April 2014
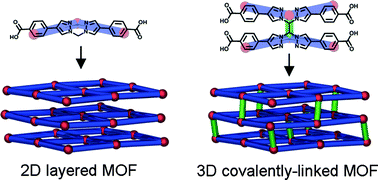
Utilising hinged ligands in MOF synthesis: a covalent linking strategy for forming 3D MOFs
Campbell J. Coghlan, Christopher J. Sumby and Christian J. Doonan
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE00181H
Free to access until 28th April 2014
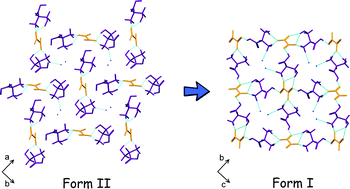
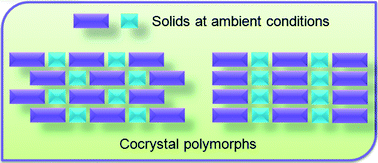
Polymorphism in cocrystals: a review and assessment of its significance
Srinivasulu Aitipamula, Pui Shan Chow and Reginald B. H. Tan
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE42008F
Free to access until 28th April 2014












 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently, she is writing a book on chemicals from plants
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently, she is writing a book on chemicals from plants